Chemistry is the scientific study of matter, its properties, composition, structure, and the changes it undergoes. It is a fundamental discipline that plays a crucial role in understanding the world around us. Here are some key points about chemistry:
Chemistry is a dynamic field that continues to advance our understanding of the world and drive technological innovations. It provides a foundation for other scientific disciplines and offers insights into the fundamental principles governing matter and its transformations.
Chemistry is a diverse and essential field that has a wide scope and significant importance in various aspects of life. Here are some key points highlighting the scope and importance of chemistry:
Chemistry is a branch of science that explores the properties, composition, structure, and behavior of matter. It is built upon several fundamental concepts that provide a framework for understanding the subject. Here are some basic concepts of chemistry:
These are some of the basic concepts that form the foundation of chemistry. Understanding these concepts is crucial for further exploration and comprehension of more complex chemical principles and phenomena.
The molecular formula represents the actual number of atoms of each element in a molecule of a compound. It provides the specific ratio of atoms in the compound. For example, the molecular formula of glucose is C6H12O6, which indicates that each molecule of glucose contains six carbon atoms, twelve hydrogen atoms, and six oxygen atoms.
Molecular mass refers to the sum of the atomic masses of all the atoms in a molecule. It is calculated by adding up the atomic masses of the elements in the molecular formula. The unit of molecular mass is atomic mass units (amu) or grams per mole (g/mol). For example, the molecular mass of glucose (C6H12O6) can be calculated as follows:
C (carbon) = 12.01 amu
H (hydrogen) = 1.008 amu
O (oxygen) = 16.00 amu
Total molecular mass of glucose = (6 * 12.01) + (12 * 1.008) + (6 * 16.00) = 180.18 amu
The percentage composition of elements in a compound indicates the relative proportion of each element‘s mass in the compound. It is expressed as a percentage. To calculate the percentage composition, you divide the mass of each element by the total mass of the compound and multiply by 100%. For example, in glucose (C6H12O6), you can determine the percentage composition as follows:
Mass of carbon = 6 * 12.01 amu = 72.06 amu
Mass of hydrogen = 12 * 1.008 amu = 12.096 amu
Mass of oxygen = 6 * 16.00 amu = 96.00 amu
Total mass of glucose = 180.18 amu
Percentage composition of carbon = (72.06 amu / 180.18 amu) * 100% = 40.00%
Percentage composition of hydrogen = (12.096 amu / 180.18 amu) * 100% = 6.72%
Percentage composition of oxygen = (96.00 amu / 180.18 amu) * 100% = 53.28%
The empirical formula represents the simplest, most reduced ratio of elements in a compound. It indicates the relative number of atoms of each element present in a compound. The empirical formula is derived from the molecular formula by dividing all the subscripts by their greatest common divisor. For example, the empirical formula of glucose (C6H12O6) is CH2O, as the ratio of carbon, hydrogen, and oxygen atoms is 1:2:1.
These concepts are fundamental to understanding the composition, structure, and properties of chemical compounds.
Chemical change refers to a process in which one or more substances are transformed into new substances with different chemical properties. During a chemical change, the arrangement of atoms in molecules or compounds is altered, resulting in the formation of new chemical bonds and the breaking of existing bonds. Some common examples of chemical changes include combustion, oxidation, and chemical reactions. Characteristics of a chemical change include the formation of new substances, changes in color, production of gas, release or absorption of energy (heat or light), and irreversibility.
A chemical equation is a symbolic representation of a chemical reaction. It uses chemical formulas and symbols to describe the reactants, products, and their relative proportions in a balanced manner. A balanced chemical equation shows the conservation of mass, meaning that the total number of atoms of each element is the same on both sides of the equation. The reactants are written on the left side, separated by a plus sign (+), and the products are written on the right side, separated by an arrow (→) indicating the direction of the reaction. Coefficients are used to balance the equation by adjusting the number of molecules or moles of each substance involved.
For example, the combustion of methane (CH4) can be represented by the following balanced chemical equation:
CH4 + 2O2
This equation shows that one molecule of methane reacts with two molecules of oxygen gas to produce one molecule of carbon dioxide and two molecules of water.
Chemical equations are important tools in chemistry as they provide a concise and standardized way of representing chemical reactions. They allow scientists to communicate and understand the changes that occur during chemical processes and enable the prediction of products and stoichiometric calculations.
Dalton‘s atomic theory, proposed by John Dalton in the early 19th century, laid the foundation for our understanding of atoms and their behavior. The key postulates of Dalton‘s atomic theory are:
| Law | Description | Example |
|---|---|---|
Law of Conservation of Mass | The total mass of the reactants in a chemical reaction is equal to the total mass of the products. | Example: When hydrogen gas (H2) combines with oxygen gas (O2) to form water (H2O), the total mass of the reactants (hydrogen and oxygen) is equal to the total mass of the product (water). |
Law of Definite Proportions | A compound always contains the same elements in the same fixed proportions by mass. | Example: Water (H2O) always contains two hydrogen atoms for every one oxygen atom, regardless of the source of water. |
Law of Multiple Proportions | When two elements combine to form different compounds, the masses of one element that combine with a fixed mass of the other element are in a ratio of small whole numbers. | Example: Carbon and oxygen can combine to form carbon monoxide (CO) and carbon dioxide (CO2). In carbon monoxide, the mass ratio of carbon to oxygen is 1:1, while in carbon dioxide, the mass ratio of carbon to oxygen is 1:2. |
Law of Combining Volumes | When gases react, the volumes of the gases involved in the reaction are in simple whole number ratios. | Example: Hydrogen gas (H2) reacts with chlorine gas (Cl2) to form hydrogen chloride gas (HCl). The volume ratio of hydrogen to chlorine to hydrogen chloride is 1:1:2. |
Gay-Lussac‘s Law of Combining Volumes | Under the same conditions of temperature and pressure, the volumes of gases involved in a chemical reaction are in simple whole number ratios. | Example: When ammonia (NH3) reacts with hydrochloric acid (HCl) to form ammonium chloride (NH4Cl), the volume ratio of ammonia to hydrochloric acid to ammonium chloride is 1:3:2. |
Understanding atomic structure and the laws of stoichiometry is fundamental to comprehend the behavior and interactions of matter at the atomic level.
Avogadro‘s hypothesis, proposed by Amedeo Avogadro in 1811, states that equal volumes of gases, at the same temperature and pressure, contain an equal number of molecules.
Avogadro‘s hypothesis and its deductions have revolutionized our understanding of atomic and molecular behavior, providing essential tools for quantitative analysis in chemistry.
The mole concept is a fundamental concept in chemistry that allows us to quantify and relate the amounts of substances in chemical reactions. It is based on the concept of a mole, which is a unit used to measure the amount of a substance. The mole is defined as the amount of a substance that contains the same number of particles (atoms, molecules, or ions) as there are atoms in exactly 12 grams of carbon-12, which is known as Avogadro‘s number (6.022 × 10^23).
The mole concept allows us to relate the mass of a substance to the number of moles it contains. The molar mass of a substance is the mass of one mole of that substance and is expressed in grams/mole. It can be calculated by summing the atomic masses of all the atoms in a molecule or the formula unit of a compound. The relationship between mass, moles, and molar mass is given by the equation:
Mass = Number of Moles × Molar Mass
The mole concept also relates the volume of a gas to the number of moles it contains. At standard temperature and pressure (STP), one mole of any gas occupies a volume of approximately 22.4 liters. This volume is known as the molar volume of a gas at STP.
The mole concept allows us to relate the number of moles of a substance to the number of particles it contains. One mole of any substance contains Avogadro‘s number (6.022 × 10^23) of particles. This relationship is expressed by the equation:
Number of Particles = Number of Moles × Avogadro‘s Number
The mole concept is essential for performing stoichiometric calculations, determining empirical and molecular formulas, and understanding the quantitative aspects of chemical reactions. It provides a bridge between the microscopic world of atoms and molecules and the macroscopic world of measurable quantities such as mass, volume, and particles.
The empirical formula of a compound represents the simplest, most reduced ratio of the elements present in the compound. It can be determined from the percentage composition of the elements in the compound. Here‘s the general procedure:
For example, let‘s consider a compound with the following percentage composition:
| Element | Percentage |
|---|---|
| Carbon (C) | 40% |
| Hydrogen (H) | 6.7% |
| Oxygen (O) | 53.3% |
By converting the percentages to grams and then to moles, we can determine the mole ratios:
Dividing each mole value by the smallest mole value (3.33 moles), we obtain the ratio:
Thus, the empirical formula for the compound is CH2O, representing the simplest ratio of atoms in the compound.
The molecular formula of a compound represents the actual number of atoms of each element present in one molecule of the compound. It can be determined if the molar mass of the compound is known in addition to the empirical formula. Here‘s how to calculate the molecular formula:
For example, if the molar mass of the compound in the previous example is determined to be 60 g/mol, and the empirical formula mass is found to be 30 g/mol, then:
Molar mass of compound / Empirical formula mass = 60 g/mol / 30 g/mol = 2
Since the whole number obtained is 2, we multiply the subscripts of the empirical formula (CH2O) by 2, resulting in the molecular formula C2H4O2.
Thus, the molecular formula for the compound is C2H4O2, indicating that there are two carbon atoms, four hydrogen atoms, and two oxygen atoms in one molecule of the compound.
In stoichiometry, the mole concept is used to determine the quantitative relationships between reactants and products in a chemical reaction. It involves the use of balanced chemical equations and the conversion of moles to masses, volumes, or number of particles. Here‘s how to perform calculations using the mole concept in stoichiometry:
Let‘s consider an example to illustrate the calculation in a stoichiometric chemical equation:
Example:
Balance the following chemical equation and calculate the mass of carbon dioxide (CO2) produced when 5 moles of methane (CH4) react:
CH4+ 2O2
Solution:
Therefore, when 5 moles of methane react, the mass of carbon dioxide produced is 220.05 grams.
In chemical reactions, the limiting reactant is the reactant that is completely consumed, thereby limiting the amount of product that can be formed. The excess reactant is the reactant that remains unconsumed after the limiting reactant is completely reacted. The theoretical yield is the maximum amount of product that can be obtained based on the stoichiometry of the balanced chemical equation. Calculations involving limiting reactant, excess reactant, and theoretical yield are important in determining the efficiency of a reaction and predicting the amount of product that can be obtained.
The following steps can be followed to perform calculations involving limiting reactant, excess reactant, and theoretical yield:
Let‘s consider an example to illustrate calculations involving limiting reactant, excess reactant, and theoretical yield:
Example:
In the reaction between 8 moles of hydrogen gas (H2) and 6 moles of oxygen gas (O2), determine the limiting reactant, the excess reactant, and the theoretical yield of water (H2O) that can be formed.
2H2+ O2
Solution:
Therefore, in the given reaction, the limiting reactant is H2, the excess reactant is O2, and the theoretical yield of water that can be formed is 4 moles or a corresponding mass based on the molar mass of H2O.
| Atomic Structure History |
|---|
The understanding of atomic structure has evolved over centuries through the contributions of various scientists. Here is a brief overview of the major milestones in the history of atomic structure:Democritus (5th century BCE):Proposed the concept of atoms as indivisible and indestructible particles that make up all matter.John Dalton (1803):Developed Dalton‘s Atomic Theory, which stated that atoms are the smallest particles of matter and are indivisible. He also introduced the concept of chemical elements and their combinations to form compounds.J.J. Thomson (1897):Discovered the electron through his experiments on cathode rays. Proposed the Plum Pudding Model of the atom, where electrons are embedded in a positively charged sphere.Ernest Rutherford (1911):Conducted the gold foil experiment and established the nuclear model of the atom. He discovered that most of the atom‘s mass and positive charge are concentrated in a tiny, dense nucleus, while electrons orbit around it.James Chadwick (1932):Confirmed the existence of neutrons, which are neutral particles located in the atomic nucleus.These discoveries laid the foundation for our understanding of atomic structure and paved the way for further advancements in the field of atomic and nuclear physics. |
| Particle | Symbol | Charge | Relative Mass |
|---|---|---|---|
| Electron | e- | -1 | 1/1836 |
| Proton | p+ | +1 | 1 |
| Neutron | n | 0 | 1 |
The atomic structure consists of three fundamental particles: electrons, protons, and neutrons. Here are their characteristics:
These particles play crucial roles in determining the properties and behavior of atoms, as well as the formation of chemical bonds and interactions between atoms.
| Term | Definition |
|---|---|
| Nucleon | A nucleon refers to either a proton or a neutron, which are the particles present in the atomic nucleus. |
| Charge | Charge refers to the electrical property of particles. Protons have a positive charge of +1, electrons have a negative charge of -1, and neutrons have no charge (neutral). |
| Mass Number | The mass number of an atom represents the total number of protons and neutrons in its nucleus. It is denoted by the symbol ‘A‘. |
| Alpha Particles | Alpha particles are clusters of two protons and two neutrons. They are emitted during certain types of radioactive decay and have a charge of +2. |
| Isotopes | Isotopes are atoms of the same element that have the same number of protons but different numbers of neutrons. They have similar chemical properties but differ in atomic mass. |
| Isotopic and Relative Mass | Isotopic mass refers to the actual mass of a particular isotope of an element. Relative mass, on the other hand, is the mass of an isotope relative to the mass of the carbon-12 isotope, which is assigned a mass of exactly 12 atomic mass units (u). |
| Isotopic Effect | The isotopic effect refers to the differences in physical or chemical properties observed due to variations in the isotopic composition of an element. Isotopes may exhibit different reactivity, stability, or other characteristics. |
| Isobars | Isobars are atoms or ions that have the same mass number but different atomic numbers. In other words, they have the same total number of nucleons but different numbers of protons and neutrons. |
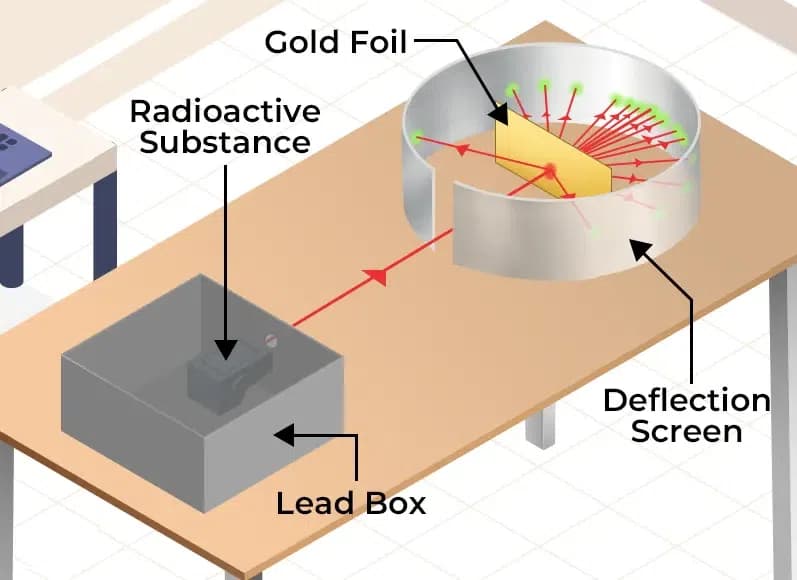
Rutherford‘s Alpha Ray Scattering Experiment and his atomic model were pivotal in advancing our understanding of atomic structure, even though subsequent discoveries and theories refined our knowledge further.
Bohr‘s Atomic Model, proposed by Niels Bohr in 1913, built upon Rutherford‘s model and provided a more detailed understanding of atomic structure. It introduced the concept of energy levels or orbits in which electrons revolve around the nucleus. Here are the key features ofBohr‘s Atomic Model:
The nucleus contains protons, which carry positive charge, and neutrons, which have no charge. Electrons, which are negatively charged, revolve around the nucleus in distinct energy levels.
Bohr‘s model explained the line spectra observed in atomic emission or absorption experiments. When an electron moves from a higher energy level to a lower energy level, it emits a photon of specific energy, resulting in a spectral line. The energy of the emitted or absorbed photons corresponds to the difference in energy between the initial and final energy levels.
Despite its limitations, Bohr‘s Atomic Model laid the foundation for further developments in quantum mechanics and led to a deeper understanding of atomic structure and behavior.
The hydrogen spectrum refers to the pattern of electromagnetic radiation emitted or absorbed by hydrogen atoms. It played a significant role in the development of quantum mechanics and provided crucial insights into the nature of atomic structure. Here are the key features of the hydrogen spectrum:
When an electron in a hydrogen atom transitions from a higher energy level to a lower energy level, it releases energy in the form of photons. These photons have specific wavelengths corresponding to different energy differences between the energy levels. The emission spectrum of hydrogen can be described using the formula:
where ΔE is the energy difference, Efinaland Einitialare the energies of the final and initial states, and nfinaland ninitialare the principal quantum numbers of the final and initial states, respectively.
The hydrogen spectrum can be divided into several series, named after the scientists who discovered them. The prominent series include:
The hydrogen spectrum provided evidence for the quantization of energy levels in atoms. The observed discrete lines in the spectrum indicated that only specific energy transitions were allowed, corresponding to specific differences in energy levels.
Bohr‘s Atomic Model successfully explained the hydrogen spectrum by incorporating the concept of quantized energy levels and electron transitions. The model predicted the wavelengths of the spectral lines based on the energy differences between the energy levels.
The study of the hydrogen spectrum and its interpretation played a crucial role in the development of quantum mechanics and our understanding of atomic structure, leading to significant advancements in physics and chemistry.
Wave-particle duality is a fundamental concept in quantum mechanics that suggests that particles, such as electrons and photons, can exhibit both wave-like and particle-like properties. This duality was first proposed by Louis de Broglie, who introduced the de Broglie equation to describe the wave nature of particles.
The de Broglie equation relates the wavelength (λ) of a particle to its momentum (p) and Planck‘s constant (h). The equation is given by:
where λ is the wavelength, h is Planck‘s constant (approximately 6.626 x 10^-34 J·s), and p is the momentum of the particle.
The de Broglie equation suggests that all particles, including matter particles, have wave-like characteristics. The wavelength associated with a particle is inversely proportional to its momentum. This means that particles with higher momentum have shorter wavelengths, while particles with lower momentum have longer wavelengths.
The de Broglie equation has several important applications in quantum mechanics:
The concept of wave-particle duality revolutionized our understanding of the microscopic world. It highlighted that particles can exhibit wave-like properties and waves can exhibit particle-like properties. This duality is fundamental to quantum mechanics and provides a deeper understanding of the behavior of particles at the atomic and subatomic levels.
The de Broglie equation is a key tool in understanding and predicting the wave-like behavior of particles and has contributed significantly to the development of modern physics.
Heisenberg‘s Uncertainty Principle is a fundamental principle in quantum mechanics that states that it is impossible to simultaneously determine certain pairs of physical properties with absolute precision. It was formulated by German physicist Werner Heisenberg in 1927.
The uncertainty principle is mathematically expressed as:
where Δx represents the uncertainty in the position of a particle, Δp represents the uncertainty in its momentum, and h is Planck‘s constant.
This principle implies that the more precisely one property (e.g., position) is measured, the less precisely the other property (e.g., momentum) can be known, and vice versa.
Heisenberg‘s Uncertainty Principle revolutionized our understanding of the nature of reality at the quantum level. It fundamentally changed the way we perceive and study particles and their properties. The principle underscores the limitations and uncertainties inherent in the measurement of quantum systems and highlights the probabilistic nature of quantum mechanics.
Heisenberg‘s Uncertainty Principle is a cornerstone of quantum theory and has shaped our modern understanding of the microscopic world.
The concept of probability plays a fundamental role in understanding
According to the uncertainty principle, the more precisely we try to measure the position of a particle, the less precisely we can know its momentum, and vice versa. This introduces an element of uncertainty or probability into our knowledge of the particle‘s properties.
In the context of Heisenberg‘s Uncertainty Principle, probability comes into play because the exact position and momentum of a particle cannot be determined with absolute certainty. Instead, we can only assign probabilities to different outcomes.
The uncertainty principle reflects the
In other words, the uncertainty principle implies that there is an inherent uncertainty or probability associated with the position and momentum of particles at the quantum level. It highlights the limitations of classical determinism and emphasizes the probabilistic nature of quantum mechanics.
Overall, the concept of probability is essential in understanding Heisenberg‘s Uncertainty Principle as it helps us grasp the inherent uncertainties and probabilistic nature of the properties of particles at the quantum level.
In atomic physics, the concepts of electron orbit and atomic orbital are used to describe the arrangement of electrons around an atomic nucleus.
An electron orbit refers to the path followed by an electron as it revolves around the nucleus of an atom. This concept was proposed by the early atomic models, such as the Bohr model. According to these models, electrons occupy specific circular orbits or energy levels at fixed distances from the nucleus.
However, the concept of electron orbits has been superseded by the more accurate atomic orbital model, based on quantum mechanics.
An atomic orbital represents the probability distribution of finding an electron within a particular region of space around the nucleus of an atom. Atomic orbitals are described by wavefunctions, which are mathematical functions that characterize the behavior of electrons in quantum mechanics.
Atomic orbitals are categorized into different shells and subshells:
For example, the first shell (n=1) has only one subshell, the s subshell. It can hold a maximum of 2 electrons. The second shell (n=2) has two subshells: the s subshell (2 electrons) and the p subshell (6 electrons).
The arrangement of electrons in atomic orbitals follows specific rules, such as the Aufbau principle, Pauli exclusion principle, and Hund‘s rule, which govern the filling order and electron configurations in atoms.
Overall, the concept of electron orbit and atomic orbital provides a framework for understanding the distribution of electrons in atoms and the organization of energy levels, shells, and subshells.
In quantum mechanics, quantum numbers are used to describe the characteristics and properties of electrons in an atom. They provide a way to identify and differentiate the various atomic orbitals and their corresponding electrons. There are four main quantum numbers: principal quantum number (n), azimuthal quantum number (l), magnetic quantum number (ml), and spin quantum number (ms).
The principal quantum number (n) indicates the energy level or shell in which an electron resides. It represents the average distance of the electron from the nucleus. The values of n are positive integers (1, 2, 3, ...) starting from 1. Higher values of n correspond to higher energy levels and larger orbitals. For example, the first shell has n=1, the second shell has n=2, and so on.
The azimuthal quantum number (l) determines the shape of the atomic orbital. It represents the subshell or orbital type within a particular shell. The values of l range from 0 to (n-1). Each value of l corresponds to a specific subshell: 0 represents the s subshell, 1 represents the p subshell, 2 represents the d subshell, and 3 represents the f subshell.
The magnetic quantum number (ml) specifies the orientation or spatial orientation of the atomic orbital within a subshell. It determines the number of orbitals within a subshell. The values of mlrange from -l to +l, including zero. For example, for the p subshell (l=1), the possible values of mlare -1, 0, and +1, indicating the three perpendicular p orbitals (px, py, pz).
The spin quantum number (ms) represents the intrinsic angular momentum or spin of the electron. It describes the two possible spin orientations of an electron: spin-up (+1/2) or spin-down (-1/2).
The combination of these quantum numbers provides a unique set of values for each electron in an atom, defining its energy level, orbital shape, spatial orientation, and spin orientation.
Quantum numbers are crucial for understanding electron configurations, determining the arrangement of electrons in orbitals, and predicting the behavior and properties of atoms.
The Aufbau Principle is a fundamental principle in chemistry that describes the order in which electrons fill atomic orbitals in an atom. According to this principle, electrons occupy the lowest energy orbitals available before filling higher energy orbitals. The Aufbau Principle is based on the idea that electrons seek to attain the most stable arrangement by occupying the lowest energy levels first.
The order of filling atomic orbitals follows the sequence of increasing energy levels, known as the building-up order. The order of filling orbitals is as follows:
The Aufbau Principle helps in determining the electron configurations of atoms and understanding the distribution of electrons in different energy levels and orbitals.
The Simmon‘s (n+1) Rule, also known as the Madelung Rule, is a modification of the Aufbau Principle that takes into account the different energies of subshells within a given principal energy level. According to this rule, the order of filling orbitals within a given principal energy level is determined by the sum of the principal quantum number (n) and the azimuthal quantum number (l).
In the (n+1) Rule, the subshells are filled in the order of increasing (n+l) values. If two or more subshells have the same (n+l) value, they are filled based on the order of increasing n. The (n+1) Rule helps in determining the sequence of filling orbitals in complex atoms where subshells of different principal quantum numbers overlap.
The Simmon‘s (n+1) Rule provides a more accurate description of electron filling order compared to the simple Aufbau Principle, especially for elements with multiple electron shells and subshells.
Both the Aufbau Principle and the Simmon‘s (n+1) Rule are important for understanding electron configurations, predicting chemical properties, and explaining the periodic trends in the periodic table.
Pauli‘s Exclusion Principle is a fundamental principle in quantum mechanics that states that no two electrons in an atom can have the same set of quantum numbers. It was formulated by the Austrian physicist Wolfgang Pauli in 1925.
According to the principle, each electron within an atom is described by a unique set of quantum numbers, including the principal quantum number (n), azimuthal quantum number (l), magnetic quantum number (ml), and spin quantum number (ms). The spin quantum number specifies the orientation of the electron‘s spin and can have two possible values: +½ (spin-up) and -½ (spin-down).
The main consequence of Pauli‘s Exclusion Principle is that it leads to the filling of atomic orbitals with a maximum of two electrons, with opposite spins. This principle explains the observed electron configurations in atoms and the arrangement of electrons in different energy levels and subshells.
Pauli‘s Exclusion Principle has significant implications for the stability and chemical behavior of atoms. It helps in determining the electronic structure of elements, understanding the periodic table, and predicting the formation of chemical bonds.
Overall, Pauli‘s Exclusion Principle is a fundamental principle in quantum mechanics that governs the behavior and arrangement of electrons in atoms, providing a key foundation for our understanding of atomic structure and chemical properties.
Hund‘s Rule is a principle in quantum mechanics that describes the distribution of electrons in orbitals within a subshell. It was formulated by the German physicist Friedrich Hund in 1927.
According to Hund‘s Rule, when orbitals of equal energy, known as degenerate orbitals, are available, electrons will occupy separate orbitals with parallel spins before pairing up. This means that electrons will first fill all available orbitals in a subshell with the same spin direction (spin-up) before pairing with opposite spin (spin-down).
Hund‘s Rule can be understood based on the concept of electron-electron repulsion. Electrons are negatively charged particles, and they repel each other. By occupying separate orbitals with parallel spins, they minimize their repulsion and achieve a more stable arrangement.
This rule is particularly significant when filling the orbitals of p, d, and f subshells, which have multiple degenerate orbitals. The application of Hund‘s Rule leads to a set of half-filled and fully-filled subshells, which are relatively more stable than partially filled subshells.
Hund‘s Rule plays a crucial role in determining the electron configurations of atoms, especially for elements beyond the first few in the periodic table. It helps explain the observed distribution of electrons in subshells and the resulting magnetic properties of atoms and ions.
Overall, Hund‘s Rule of Maximum Multiplicity provides insights into the arrangement of electrons within subshells and contributes to our understanding of atomic structure and the behavior of electrons in atoms.
Electronic configuration refers to the arrangement of electrons within an atom or ion in different energy levels, subshells, and orbitals. It describes the distribution of electrons according to their quantum numbers, following the principles and rules of quantum mechanics.
The electronic configuration of an atom is represented using a notation that includes the symbol of the element followed by a series of numbers and letters. The numbers indicate the principal quantum number (n), which represents the energy level or shell, while the letters represent the subshell or orbital type (s, p, d, f).
The rules and principles used to determine the electronic configuration of atoms are as follows:
| Element | Symbol | Electronic Configuration |
|---|---|---|
| Hydrogen | H | 1s1 |
| Helium | He | 1s2 |
| Lithium | Li | 1s22s1 |
| Beryllium | Be | 1s22s2 |
| Boron | B | 1s22s22p1 |
| Carbon | C | 1s22s22p2 |
| Nitrogen | N | 1s22s22p3 |
| Oxygen | O | 1s22s22p4 |
| Fluorine | F | 1s22s22p5 |
| Neon | Ne | 1s22s22p6 |
| Sodium | Na | 1s22s22p63s1 |
| Magnesium | Mg | 1s22s22p63s2 |
| Aluminum | Al | 1s22s22p63s23p1 |
| Silicon | Si | 1s22s22p63s23p2 |
| Phosphorus | P | 1s22s22p63s23p3 |
| Sulfur | S | 1s22s22p63s23p4 |
| Chlorine | Cl | 1s22s22p63s23p5 |
| Argon | Ar | 1s22s22p63s23p6 |
| Potassium | K | 1s22s22p63s23p64s1 |
| Calcium | Ca | 1s22s22p63s23p64s2 |
| Scandium | Sc | 1s22s22p63s23p64s23d1 |
| Titanium | Ti | 1s22s22p63s23p64s23d2 |
| Vanadium | V | 1s22s22p63s23p64s23d3 |
| Chromium | Cr | 1s22s22p63s23p64s13d5 |
| Manganese | Mn | 1s22s22p63s23p64s23d5 |
| Iron | Fe | 1s22s22p63s23p64s23d6 |
| Cobalt | Co | 1s22s22p63s23p64s23d7 |
| Nickel | Ni | 1s22s22p63s23p64s23d8 |
| Copper | Cu | 1s22s22p63s23p64s13d10 |
| Zinc | Zn | 1s22s22p63s23p64s23d10 |
The electronic configuration provides important information about the organization of electrons in an atom, including the number of electrons in each energy level, subshell, and orbital. It helps in understanding the chemical properties, reactivity, and bonding behavior of elements.
For example, the electronic configuration of oxygen (O) is 1s^2 2s^2 2p^4, indicating that it has two electrons in the 1s orbital, two in the 2s orbital, and four in the 2p orbital.
Overall, the electronic configuration is a fundamental concept in chemistry that helps in explaining the behavior and properties of elements and their interactions with other substances.
Joseph Louis Proust contributed to the development of chemical classification with his Law of Definite Proportions. This law states that chemical compounds always contain the same elements in the same fixed proportions by mass. In other words, the ratio of elements in a compound is constant regardless of the amount of the compound present.
Johann Wolfgang Döbereiner proposed the concept of triads, where he grouped elements into sets of three based on their similar chemical properties. He observed that the atomic weight of the middle element in a triad was approximately the average of the other two elements. For example, the triad of lithium, sodium, and potassium exhibited similar chemical behavior, and the atomic weight of sodium was the average of lithium and potassium.
John Newlands arranged the known elements in order of increasing atomic weight and noticed that properties repeated every eighth element, similar to the musical octave. This periodicity led to the Law of Octaves, which stated that every eighth element had similar chemical properties. Newlands‘ classification was significant as it was the first attempt to organize elements based on periodicity.
Dmitri Mendeleev is widely credited for developing the modern periodic table. He arranged the elements in order of increasing atomic weight and observed that elements with similar properties occurred at regular intervals. Mendeleev left gaps in his periodic table for undiscovered elements and accurately predicted their properties. He also emphasized the periodicity of elements and proposed the concept of periodic law, stating that the properties of elements are a periodic function of their atomic weights.
The Modern Periodic Law states that the physical and chemical properties of elements are periodic functions of their atomic numbers. This means that when elements are arranged in order of increasing atomic number, their properties repeat in a regular pattern. The modern periodic law builds upon Mendeleev‘s periodic law and takes into account the concept of atomic numbers rather than atomic weights.
The modern periodic table is a tabular arrangement of elements based on their atomic numbers, electronic configurations, and chemical properties. It is divided into periods (horizontal rows) and groups (vertical columns). Each element is represented by its atomic symbol, atomic number, and atomic mass. The elements in the periodic table are arranged in such a way that elements with similar properties are placed in the same group.
The modern periodic table consists of blocks, including the s-block, p-block, d-block, and f-block, based on the electron configuration of elements. The table provides valuable information about the properties and characteristics of elements, including their reactivity, atomic size, ionization energy, and electronegativity.
The modern periodic table has been expanded to include the discovery of new elements beyond atomic number 118. It has undergone revisions and refinements over time to accommodate the understanding of the properties and behaviors of elements.
| Periodic Table | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H 1 1.0079 | He 2 4.0026 | Li 3 6.941 | Be 4 9.0122 | B 5 10.81 | C 6 12.011 | N 7 14.007 | O 8 15.999 | F 9 18.998 | Ne 10 20.18 | Na 11 22.99 | Mg 12 24.305 | Al 13 26.982 | Si 14 28.085 | P 15 30.974 | S 16 32.06 | Cl 17 35.45 | Ar 18 39.948 |
| K 19 39.098 | Ca 20 40.078 | Sc 21 44.956 | Ti 22 47.867 | V 23 50.942 | Cr 24 51.996 | Mn 25 54.938 | Fe 26 55.845 | Co 27 58.933 | Ni 28 58.693 | Cu 29 63.546 | Zn 30 65.38 | Ga 31 69.723 | Ge 32 72.63 | As 33 74.922 | Se 34 78.96 | Br 35 79.904 | Kr 36 83.798 |
| Rb 37 85.468 | Sr 38 87.62 | Y 39 88.906 | Zr 40 91.224 | Nb 41 92.906 | Mo 42 95.95 | Tc 43 (98) | Ru 44 101.07 | Rh 45 102.91 | Pd 46 106.42 | Ag 47 107.87 | Cd 48 112.41 | In 49 114.82 | Sn 50 118.71 | Sb 51 121.76 | Te 52 127.6 | I 53 126.9 | Xe 54 131.29 |
| Cs 55 132.91 | Ba 56 137.33 | La-Lu | Hf 72 178.49 | Ta 73 180.95 | W 74 183.84 | Re 75 186.21 | Os 76 190.23 | Ir 77 192.22 | Pt 78 195.08 | Au 79 196.97 | Hg 80 200.59 | Tl 81 204.38 | Pb 82 207.2 | Bi 83 208.98 | Po 84 (209) | At 85 (210) | Rn 86 (222) |
| Period | Group | Element | Symbol | Atomic Number | Atomic Mass |
|---|---|---|---|---|---|
| 1 | 1 | Hydrogen | H | 1 | 1.008 |
| 1 | 2 | Helium | He | 2 | 4.0026 |
| 2 | 1 | Lithium | Li | 3 | 6.941 |
| 2 | 2 | Beryllium | Be | 4 | 9.0122 |
| 2 | 3 | Boron | B | 5 | 10.811 |
| 2 | 4 | Carbon | C | 6 | 12.011 |
| 2 | 5 | Nitrogen | N | 7 | 14.007 |
| 2 | 6 | Oxygen | O | 8 | 15.999 |
| 2 | 7 | Fluorine | F | 9 | 18.998 |
| 2 | 8 | Neon | Ne | 10 | 20.180 |
Ionization energy refers to the energy required to remove an electron from a neutral atom or ion in its gaseous state. It is a measure of the atom‘s or ion‘s ability to hold onto its electrons and resist losing them.
Key Points:
It‘s important to note that ionization energy values can vary depending on the specific element or ion and the experimental conditions.
Atomic and ionic radii refer to the size of atoms and ions, respectively. The radii are typically measured as the distance from the nucleus to the outermost electron shell or the outermost electron in an ion.
Key Points:
It‘s important to note that atomic and ionic radii can vary depending on the specific element, its oxidation state, and the coordination number in ionic compounds.
Electronegativity is a measure of an atom‘s tendency to attract electrons towards itself when participating in a chemical bond. It helps determine the polarity of bonds and molecules. Electronegativity is typically represented on the Pauling scale, which assigns arbitrary values ranging from 0.7 to 4.0 to elements.
Key Points:
It‘s important to note that electronegativity values are approximate and can vary slightly depending on the scale used.
Electron affinity refers to the energy change that occurs when an atom or ion gains an electron to form a negative ion. It is a measure of the atom‘s or ion‘s tendency to attract and bind an additional electron.
Key Points:
It‘s important to note that electron affinity values can vary depending on the specific element or ion and the experimental conditions.
Metallic character refers to the extent to which an element exhibits the properties characteristic of metals. These properties include high electrical and thermal conductivity, luster, malleability, ductility, and the tendency to form positive ions (cations) in chemical reactions.
Key Points:
It‘s important to note that metallic character is a relative concept and can vary depending on the specific element and the context in which it is considered.
Electronic Theory of Valency:
The electronic theory of valency, also known as the electron-pair theory of valency, is a concept in chemistry that explains the formation of chemical bonds and the determination of the valence (combining) capacity of atoms based on their electronic structures.
Key Points:
The electronic theory of valency has been foundational in understanding chemical bonding and the behavior of elements in the formation of compounds. It has contributed significantly to the development of modern chemistry.
Electrovalent or Ionic Bond (Electrovalency):
An electrovalent or ionic bond is a type of chemical bond formed between two atoms when there is a complete transfer of electrons from one atom to another. This results in the formation of positively charged cations and negatively charged anions, which are held together by electrostatic attraction.
Key Points:
Electrovalent or ionic bonds are important in the formation of many compounds, including salts, oxides, and many minerals. They exhibit distinctive properties and play a significant role in various chemical and biological processes.
Covalent Bond (Covalency):
A covalent bond, also known as covalency, is a type of chemical bond formed between atoms through the sharing of electrons. In a covalent bond, atoms share one or more pairs of electrons to achieve a more stable electron configuration.
Key Points:
Covalent bonds play a crucial role in organic chemistry, where complex molecules are formed through a series of covalent bond formations. They are also fundamental in understanding the structure, properties, and reactivity of a wide range of compounds found in nature and synthetic materials.
Coordinate Covalent Bond (or Dative Bond):
A coordinate covalent bond, also known as a dative bond, is a type of covalent bond in which both electrons of the shared electron pair come from the same atom. Unlike a typical covalent bond where each atom contributes one electron to the shared pair, in a coordinate covalent bond, one atom donates both electrons to the bond.
Key Points:
Coordinate covalent bonds are important in understanding the formation and behavior of complex molecules, coordination compounds, and reaction mechanisms. They contribute to the stability, reactivity, and diverse properties exhibited by many compounds in various fields of chemistry.
Lewis Structure:
Lewis structures, also known as Lewis dot structures or electron dot structures, are diagrams that represent the arrangement of electrons in a molecule or ion. They were developed by American chemist Gilbert N. Lewis in 1916 as a way to visualize the bonding and electron distribution in chemical compounds.
Key Points:
By drawing Lewis structures, chemists can determine the number and types of bonds in a compound, identify lone pairs of electrons, and analyze the overall electronic distribution. Lewis structures are a valuable tool for understanding the structure, bonding, and behavior of molecules and ions.
Resonance:
Resonance, in the context of chemistry, refers to the phenomenon where multiple Lewis structures can be drawn for a molecule or ion that differ only in the placement of electrons. These different structures are called resonance structures, and the actual structure of the molecule or ion is considered to be a hybrid or combination of these resonance forms.
Key Points:
Resonance is a concept that allows chemists to better understand the electronic structure and behavior of complex molecules and ions. It helps explain the stability, reactivity, and properties of various compounds and is an essential concept in organic chemistry.
VSEPR Theory:
The VSEPR (Valence Shell Electron Pair Repulsion) theory is a model used in chemistry to predict the shape and geometry of molecules based on the repulsion between electron pairs in the valence shell of atoms. It helps in understanding the three-dimensional arrangement of atoms in a molecule and the bond angles formed between them.
Key Points:
The VSEPR theory provides a simple yet effective approach to predict and explain the shapes of molecules. It has widespread applications in various areas of chemistry, including organic chemistry, inorganic chemistry, and biochemistry, aiding in the understanding of molecular properties and reactivity.
Valence Bond Theory:
The Valence Bond Theory is a model used in chemistry to explain chemical bonding in molecules. It focuses on the overlapping of atomic orbitals to form covalent bonds between atoms. According to this theory, the bond between atoms is formed when their atomic orbitals overlap and share electrons.
Key Points:
The Valence Bond Theory is a fundamental concept in understanding chemical bonding and the behavior of molecules. It provides a valuable framework for explaining the formation and properties of covalent bonds in various compounds.
Hybridization:
Hybridization is a concept in chemistry that describes the mixing of atomic orbitals to form hybrid orbitals, which are used to explain the geometry and bonding in molecules. It is based on the idea that atomic orbitals combine to create new hybrid orbitals that are more suitable for bonding.
Key Points:
Overall, hybridization is a powerful tool in understanding the molecular structure and bonding in various compounds. It allows chemists to predict and explain the properties and behavior of molecules, contributing to the advancement of many areas of chemistry.
Bond Characteristics:
Bond characteristics refer to the properties and features associated with chemical bonds formed between atoms in molecules or compounds. These characteristics determine the strength, polarity, and behavior of the bonds. Here are some key bond characteristics:
1. Bond Length:
Bond length is the distance between the nuclei of two bonded atoms. It is determined by the size of the atoms and the number of bonded electrons. In general, as the number of bonded electrons increases, the bond length decreases. Different types of bonds, such as single, double, and triple bonds, have different bond lengths.
2. Bond Energy:
Bond energy, also known as bond dissociation energy, is the energy required to break a chemical bond and separate the bonded atoms. It represents the strength of the bond. Bonds with higher bond energy are stronger and require more energy to break. The bond energy is influenced by factors such as bond length, bond type, and the nature of the bonded atoms.
3. Bond Polarity:
Bond polarity refers to the distribution of electron density between atoms in a bond. It is determined by the difference in electronegativity between the bonded atoms. If the electronegativity difference is significant, the bond is polar, meaning that there is an uneven distribution of charge. In nonpolar bonds, the electronegativity difference is small or negligible.
4. Bond Strength:
Bond strength is a measure of the force holding atoms together in a bond. It depends on factors such as bond length, bond energy, and the nature of the bonded atoms. Strong bonds require more energy to break and are more stable. Weak bonds are easily broken and are less stable.
5. Bond Angle:
Bond angle refers to the angle formed between two adjacent bonds in a molecule. It is determined by the arrangement of atoms and electron pairs around the central atom. Bond angles influence the molecular shape and can affect the chemical properties and reactivity of molecules.
6. Bond Dipole Moment:
Bond dipole moment is a measure of the polarity of a bond. It is the product of the bond length and the difference in electronegativity between the bonded atoms. A bond with a higher dipole moment indicates a greater separation of charge and stronger polarity.
7. Bond Order:
Bond order is the number of chemical bonds between a pair of atoms. It represents the strength and stability of the bond. A higher bond order corresponds to a stronger bond and shorter bond length.
8. Resonance:
Resonance occurs when multiple valid Lewis structures can be drawn for a molecule or ion. It indicates the delocalization of electrons and contributes to the stability of the molecule or ion.
These bond characteristics play a crucial role in determining the properties and behavior of molecules and compounds. They provide insights into the strength, stability, polarity, and reactivity of chemical bonds, which are fundamental to understanding the structure and function of matter.
Van der Waals Forces:
Van der Waals forces are a type of intermolecular force that exists between neutral atoms, molecules, or ions. These forces arise from the temporary fluctuations in electron distribution within particles, leading to the formation of temporary dipoles. There are three types of van der Waals forces:
1. London Dispersion Forces:
London dispersion forces are the weakest type of van der Waals forces. They occur between all molecules and atoms, regardless of their polarity. London dispersion forces arise from the temporary shifts in electron density, resulting in the formation of instantaneous dipoles. These temporary dipoles induce similar dipoles in neighboring particles, leading to attractive forces. The strength of London dispersion forces increases with the size and shape of the particles.
2. Dipole-Dipole Interactions:
Dipole-dipole interactions occur between polar molecules that possess permanent dipoles. These forces arise from the attraction between the positive end of one molecule and the negative end of another molecule. The strength of dipole-dipole interactions depends on the magnitude of the dipole moment and the distance between the molecules.
3. Hydrogen Bonding:
Hydrogen bonding is a special type of dipole-dipole interaction that occurs when a hydrogen atom is bonded to a highly electronegative atom (such as nitrogen, oxygen, or fluorine) and forms a bond with another electronegative atom in a different molecule. Hydrogen bonding is stronger than typical dipole-dipole interactions and plays a crucial role in various biological and chemical processes.
Van der Waals forces are responsible for various phenomena, including the condensation of gases into liquids, the formation of solids, and the interaction between molecules in biological systems. These forces contribute to the physical properties of substances, such as boiling and melting points, solubility, and viscosity.
Hydrogen Bond:
Hydrogen bonding is a special type of intermolecular force that occurs between a hydrogen atom bonded to a highly electronegative atom (such as nitrogen, oxygen, or fluorine) and another electronegative atom in a different molecule. It is a stronger type of dipole-dipole interaction and has distinct properties and effects. Here are some key points about hydrogen bonding:
1. Formation:
Hydrogen bonding occurs when a hydrogen atom is covalently bonded to a highly electronegative atom (denoted as X) and forms an electrostatic interaction with a lone pair of electrons on another electronegative atom (denoted as Y) in a different molecule. The hydrogen atom acts as a bridge between the two electronegative atoms, creating a partial positive charge on the hydrogen atom and a partial negative charge on the electronegative atom Y.
2. Strength:
Hydrogen bonds are stronger than typical dipole-dipole interactions and van der Waals forces. The strength of a hydrogen bond depends on various factors, including the electronegativity of the atoms involved, the distance between the interacting atoms, and the angle of the hydrogen bond.
3. Effects:
Hydrogen bonding has significant effects on the properties and behavior of substances. Some key effects include:
Hydrogen bonding is an essential concept in understanding the properties of many substances, ranging from water and biomolecules to chemical compounds. Its unique strength and effects make it a fundamental force in various scientific disciplines.
Metallic Bond:
Metallic bonding is a type of chemical bonding that occurs between metal atoms. It is responsible for the unique properties of metals, such as their high electrical and thermal conductivity, malleability, and ductility. Here are some key points about metallic bonding:
1. Electron Sea Model:
In metallic bonding, metal atoms release their valence electrons, forming a "sea" of delocalized electrons surrounding the positively charged metal ions. The valence electrons are not associated with any specific atom and are free to move throughout the metal lattice.
2. Electrostatic Attraction:
The positively charged metal ions and the negatively charged delocalized electrons experience a strong electrostatic attraction. The metal ions act as fixed cations in the lattice, while the delocalized electrons form a cloud of negative charge that surrounds the metal ions.
3. Properties:
Metallic bonding contributes to several characteristic properties of metals:
Overall, metallic bonding is responsible for the unique physical and chemical properties of metals. It enables the flow of electricity, the efficient transfer of heat, and the ability of metals to be shaped and formed, making them essential materials in various applications.
Classical Concept of Oxidation and Reduction:
The classical concept of oxidation and reduction is based on the transfer of electrons between reactants in a chemical reaction. Here are the key points of the classical concept:
Oxidation:
Oxidation refers to a chemical process in which a substance loses electrons. It involves an increase in the oxidation state or a decrease in the number of electrons associated with an atom, ion, or molecule. Oxidation is often accompanied by other observable changes, such as an increase in oxidation number, the addition of oxygen, or the removal of hydrogen.
For example, the reaction of iron with oxygen to form iron(III) oxide (rust) is an oxidation process:
4Fe + 3O2→2Fe2O3
In this reaction, iron (Fe) is oxidized from an oxidation state of 0 to +3, indicating a loss of electrons.
Reduction:
Reduction refers to a chemical process in which a substance gains electrons. It involves a decrease in the oxidation state or an increase in the number of electrons associated with an atom, ion, or molecule. Reduction is often accompanied by other observable changes, such as a decrease in oxidation number, the addition of hydrogen, or the removal of oxygen.
Continuing with the previous example, the reduction half-reaction can be represented as:
O2+ 4e-→2O2-
In this reaction, oxygen (O2) is reduced, as it gains four electrons to form oxide ions (O2-).
Redox Reactions:
Oxidation and reduction always occur together in chemical reactions and are collectively referred to as redox reactions. In a redox reaction, one substance is oxidized (loses electrons) while another substance is reduced (gains electrons). The substance that undergoes oxidation is called the reducing agent, as it causes the reduction of another substance. Conversely, the substance that undergoes reduction is called the oxidizing agent, as it causes the oxidation of another substance.
The classical concept of oxidation and reduction is fundamental in understanding the behavior of many chemical reactions, particularly those involving the transfer of electrons. It provides a basis for the study of redox reactions and plays a crucial role in various areas of chemistry, including electrochemistry, combustion, corrosion, and biological processes.
Electronic Concept of Redox Reaction:
The electronic concept of redox reactions focuses on the transfer of electrons between reactants, leading to changes in their oxidation states. Here are the key points of the electronic concept:
Oxidation:
In the electronic concept, oxidation is defined as the loss of electrons from a species. It occurs when an atom, ion, or molecule loses one or more electrons, resulting in an increase in its oxidation state. Oxidation is often associated with the formation of positive ions (cations) or the addition of oxygen.
For example, consider the reaction between magnesium (Mg) and oxygen (O2) to form magnesium oxide (MgO):
2Mg + O2→2MgO
In this reaction, magnesium is oxidized as it loses two electrons to form Mg2+ions with an oxidation state of +2.
Reduction:
In the electronic concept, reduction is defined as the gain of electrons by a species. It occurs when an atom, ion, or molecule accepts one or more electrons, resulting in a decrease in its oxidation state. Reduction is often associated with the formation of negative ions (anions) or the addition of hydrogen.
Continuing with the previous example, the reduction half-reaction can be represented as:
O2+ 4e-→2O2-
In this reaction, oxygen is reduced as it gains four electrons to form oxide ions (O2-).
Redox Reactions:
A redox reaction occurs when there is a simultaneous oxidation and reduction process. In a redox reaction, one species donates electrons (undergoes oxidation) while another species accepts those electrons (undergoes reduction). The species donating electrons is called the reducing agent, as it causes the reduction of another species. Conversely, the species accepting electrons is called the oxidizing agent, as it causes the oxidation of another species.
Redox reactions play a fundamental role in various chemical and biological processes, such as energy production, corrosion, and metabolism. Understanding the electronic concept of redox reactions helps in predicting and balancing chemical equations, as well as in analyzing the behavior of electron transfer reactions in different systems.
Oxidation Number:
Oxidation number, also known as oxidation state, is a concept used to describe the distribution of electrons in a compound or ion. It is a hypothetical charge assigned to an atom within a molecule or ion to indicate the apparent transfer of electrons between atoms. Here are the key points related to oxidation numbers:
Definition:
Oxidation number is a positive or negative number assigned to an atom in a compound or ion to represent its relative electron distribution. It is based on a set of rules and guidelines, rather than the actual charge on the atom.
Rules for Assigning Oxidation Numbers:
1. The oxidation number of an atom in its elemental state is zero.
2. In a neutral compound, the sum of the oxidation numbers of all atoms is zero.
3. The oxidation number of a monatomic ion is equal to its charge.
4. In most compounds, the oxidation number of hydrogen is +1, and the oxidation number of oxygen is -2.
5. The oxidation number of fluorine is always -1 in its compounds.
6. The oxidation number of alkali metals (Group 1 elements) is +1, and the oxidation number of alkaline earth metals (Group 2 elements) is +2.
7. The oxidation number of halogens (Group 17 elements) is -1, except when they are combined with more electronegative elements.
Significance of Oxidation Numbers:
Oxidation numbers help in determining the oxidation and reduction processes in redox reactions. They provide information about the electron transfer and changes in the oxidation states of atoms. The difference in oxidation numbers of atoms before and after a reaction indicates the number of electrons gained or lost, thus aiding in balancing chemical equations.
It is important to note that oxidation numbers are a formalism and do not necessarily represent the actual distribution of electrons. They are useful in understanding the electron flow and chemical behavior of compounds, particularly in redox reactions and stoichiometry.
Balancing Redox Reactions:
Redox reactions involve the transfer of electrons between species. Balancing these reactions is essential to ensure the conservation of mass and charge. Two commonly used methods for balancing redox reactions are the oxidation number method and the ion-electron method.
1. Balancing Redox Reactions using Oxidation Number Method:
The oxidation number method involves assigning oxidation numbers to each element in the reaction and adjusting their coefficients to balance the overall change in oxidation states. Here‘s a step-by-step procedure:
Step 1:Assign oxidation numbers to each atom in the reactants and products.
Step 2:Identify the elements undergoing oxidation and reduction by comparing their oxidation numbers before and after the reaction.
Step 3:Determine the change in oxidation number for each element undergoing oxidation or reduction.
Step 4:Balance the change in oxidation states by adjusting the coefficients of the reactants and products.
Step 5:Balance the remaining atoms and charges to ensure mass and charge conservation.
Example:Let‘s balance the following redox reaction using the oxidation number method:
Cr2O7^2- + Fe^2+ →Cr^3+ + Fe^3+
Step 1:Assign oxidation numbers:
| Species | Oxidation Number |
|---|---|
| Cr2O7^2- | +6 (for Cr), -2 (for O) |
| Fe^2+ | +2 |
| Cr^3+ | +3 |
| Fe^3+ | +3 |
Step 2:Identify oxidation and reduction:
The oxidation state of Cr decreases from +6 to +3 (reduction), while the oxidation state of Fe increases from +2 to +3 (oxidation).
Step 3:Determine the change in oxidation state:
Cr: +6 →+3 (change = -3)
Fe: +2 →+3 (change = +1)
Step 4:Balance the change in oxidation state:
Cr2O7^2- + 6Fe^2+ →2Cr^3+ + 6Fe^3+
Step 5:Balance remaining atoms and charges:
Add H2O molecules and H^+ ions as needed to balance the equation:
Cr2O7^2- + 6Fe^2+ + 14H^+ →2Cr^3+ + 6Fe^3+ + 7H2O
The equation is now balanced using the oxidation number method.
2. Balancing Redox Reactions using Ion-Electron Method:
The ion-electron method involves separating the redox reaction into two half-reactions: oxidation and reduction. Each half-reaction is balanced separately, considering the transfer of electrons. Here‘s a step-by-step procedure:
Step 1:Split the reaction into oxidation and reduction half-reactions.
Step 2:Balance the atoms and charges in each half-reaction, excluding the transfer of electrons.
Step 3:Balance the transfer of electrons by multiplying the half-reactions by appropriate coefficients.
Step 4:Combine the balanced half-reactions, ensuring that the number of electrons transferred is the same.
Step 5:Check the final balanced equation for mass and charge conservation.
Example:Let‘s balance the following redox reaction using the ion-electron method:
Cr2O7^2- + Fe^2+ →Cr^3+ + Fe^3+
Step 1:Split into half-reactions:
Oxidation half-reaction: Cr2O7^2- →Cr^3+
Reduction half-reaction: Fe^2+ →Fe^3+
Step 2:Balance atoms and charges in each half-reaction:
Oxidation half-reaction: Cr2O7^2- + 14H^+ →2Cr^3+ + 7H2O
Reduction half-reaction: Fe^2+ →Fe^3+ + e^-
Step 3:Balance the transfer of electrons:
Multiply the oxidation half-reaction by 6 and the reduction half-reaction by 1 to equalize the number of electrons transferred:
6Cr2O7^2- + 14H^+ →12Cr^3+ + 7H2O
6Fe^2+ →6Fe^3+ + 6e^-
Step 4:Combine the balanced half-reactions:
6Cr2O7^2- + 14H^+ + 6Fe^2+ →12Cr^3+ + 7H2O + 6Fe^3+
Step 5:Check for mass and charge conservation. The equation is now balanced using the ion-electron method.
Both the oxidation number method and the ion-electron method are commonly used for balancing redox reactions. The choice of method depends on the specific reaction and personal preference. Practice and familiarity with these methods can help in effectively balancing various types of redox reactions.
Electrolysis:
Electrolysis is a chemical process that uses an electric current to drive a non-spontaneous redox reaction. It involves the decomposition of an electrolyte into its constituent ions through the passage of electric charge. Electrolysis has both qualitative and quantitative applications in chemical analysis.
Qualitative Analysis:
In qualitative analysis, electrolysis is used to identify the presence of certain ions or compounds in a solution. It relies on the selective discharge of ions at the electrodes, leading to the formation of characteristic products. Here are a few examples:
1. Identification of Anions:
By subjecting a solution containing an unknown anion to electrolysis, specific reactions occur at the electrodes, producing distinctive products. For instance:
- Chloride ions (Cl-) produce chlorine gas (Cl2) at the anode.
- Sulfate ions (SO42-) produce oxygen gas (O2) at the anode.
- Bromide ions (Br-) produce bromine gas (Br2) at the anode.
2. Identification of Cations:
Similarly, electrolysis can be used to identify cations by observing the products formed at the electrodes. For example:
- Sodium ions (Na+) produce hydrogen gas (H2) at the cathode.
- Copper(II) ions (Cu2+) produce copper metal (Cu) at the cathode.
- Silver ions (Ag+) produce silver metal (Ag) at the cathode.
Quantitative Analysis:
In quantitative analysis, electrolysis is used to determine the amount or concentration of a particular species in a solution. It relies on Faraday‘s laws of electrolysis, which state:
First Law:The amount of substance produced or consumed during electrolysis is directly proportional to the quantity of electricity passed through the electrolyte.
Second Law:The masses of different substances produced or consumed during electrolysis are directly proportional to their respective chemical equivalents.
Based on these laws, the amount of substance can be determined by measuring the electric current and the time of electrolysis. This information, combined with the molar mass or chemical equivalent of the substance, allows for quantitative analysis.
Applications of Electrolysis:
Electrolysis has numerous applications in various fields. Here are a few notable examples:
1. Electroplating:
Electrolysis is used in electroplating to deposit a layer of metal onto a surface. This process is widely used in industries for coating objects with metals such as gold, silver, chromium, and copper.
2. Electrorefining:
In the metallurgical industry, electrolysis is employed for the purification of metals. For example, electrolytic refining is used to obtain pure copper, zinc, and aluminum from impure samples.
3. Electrolytic Cells:
Electrolytic cells are devices that use electrolysis for various applications, including the production of chlorine gas, sodium hydroxide, and hydrogen gas.
4. Electrolysis in Water Splitting:
Electrolysis plays a crucial role in the process of water splitting, where water is decomposed into hydrogen gas and oxygen gas. This method is used for the production of hydrogen as a clean and sustainable energy source.
5. Electrolysis in Analytical Chemistry:
Electrolysis is utilized in analytical chemistry for qualitative and quantitative analysis, as discussed earlier, to identify and measure the concentration of specific ions or compounds in a solution.
Overall, electrolysis has broad applications in both qualitative and quantitative analysis, as well as in various industrial processes. It offers valuable insights into the composition and behavior of substances, making it an essential technique in the field of chemistry.
Introduction to States of Matter:
Matter, in its various forms, can exist in different states known as the states of matter. The three primary states of matter are solids, liquids, and gases. Understanding the properties and behavior of these states is fundamental to the study of chemistry and physics.
Solids:
Solids are characterized by their definite shape and volume. In a solid state, particles are closely packed and have strong intermolecular forces that hold them together in a fixed arrangement. The particles vibrate around fixed positions, but their overall positions remain relatively unchanged. Solids are not easily compressible and have high densities.
Examples of solids include rocks, metals, wood, and ice.
Liquids:
Liquids do not have a definite shape but have a definite volume. The particles in a liquid have more freedom of movement compared to solids, allowing them to flow and take the shape of their container. Intermolecular forces in liquids are weaker than in solids, allowing particles to move more freely. Liquids are also not easily compressible and have intermediate densities.
Examples of liquids include water, oil, and ethanol.
Gases:
Gases have neither a definite shape nor a definite volume. Gas particles are highly mobile and move randomly in all directions, filling the entire space available to them. The intermolecular forces in gases are weak, and the particles are far apart from each other. Gases are easily compressible and have low densities.
Examples of gases include air, oxygen, and carbon dioxide.
These three states of matter can undergo phase changes when subjected to different conditions of temperature and pressure:
-Melting:The change from a solid to a liquid state, typically by increasing temperature.
-Freezing:The change from a liquid to a solid state, typically by decreasing temperature.
-Vaporization:The change from a liquid to a gas state, which includes both evaporation (at the surface) and boiling (throughout the liquid).
-Condensation:The change from a gas to a liquid state, typically by decreasing temperature.
-Sublimation:The change from a solid directly to a gas state, or vice versa, without going through the liquid state.
The behavior of matter in each state is governed by the kinetic theory of matter, which explains the motion and interactions of particles at the molecular level.
Understanding the different states of matter and their properties is crucial in various fields of science and technology, including chemistry, physics, materials science, and engineering.
Kinetic Molecular Theory of Gases:
The kinetic molecular theory is a model that explains the behavior of gases based on the motion and interactions of their individual particles. The key postulates of the kinetic molecular theory are:
The kinetic molecular theory provides a basis for understanding the macroscopic properties and behavior of gases.
Boyle‘s Law:
Boyle‘s Law states that at a constant temperature, the volume of a given amount of gas is inversely proportional to its pressure. Mathematically, it can be expressed as:
P1V1 = P2V2
Where P1 and V1 are the initial pressure and volume, and P2 and V2 are the final pressure and volume.
Boyle‘s Law shows that as the pressure on a gas increases, its volume decreases, and vice versa, as long as the temperature remains constant.
Charles‘s Law:
Charles‘s Law states that at a constant pressure, the volume of a given amount of gas is directly proportional to its absolute temperature. Mathematically, it can be expressed as:
V1/T1 = V2/T2
Where V1 and T1 are the initial volume and temperature (in Kelvin), and V2 and T2 are the final volume and temperature.
Charles‘s Law shows that as the temperature of a gas increases, its volume also increases, and vice versa, as long as the pressure remains constant.
Avogadro‘s Law:
Avogadro‘s Law states that equal volumes of gases, at the same temperature and pressure, contain an equal number of particles (molecules or atoms). Mathematically, it can be expressed as:
V1/n1 = V2/n2
Where V1 and n1 are the initial volume and number of particles, and V2 and n2 are the final volume and number of particles.
Avogadro‘s Law implies that at the same temperature and pressure, the ratio of volumes of gases is equal to the ratio of their respective numbers of particles.
Combined Gas Equation or Ideal Gas Equation:
The combined gas equation combines Boyle‘s Law, Charles‘s Law, and Avogadro‘s Law into a single equation. It is commonly known as the ideal gas equation and is expressed as:
PV = nRT
Where P is the pressure, V is the volume, n is the number of moles of gas, R is the ideal gas constant, and T is the temperature (in Kelvin).
The ideal gas equation allows the calculation of various properties of gases, such as pressure, volume, temperature, and number of moles, under different conditions.
Partial Pressure:
Partial pressure is the pressure exerted by an individual gas in a mixture of gases. According to Dalton‘s Law of Partial Pressures, the total pressure exerted by a mixture of non-reacting gases is equal to the sum of the partial pressures of the individual gases.
The partial pressure of a gas can be calculated using the following equation:
Partial Pressure = Total Pressure x Mole Fraction
Where the mole fraction is the ratio of the number of moles of a particular gas to the total number of moles in the mixture.
Diffusion and Graham‘s Law:
Diffusion is the spontaneous mixing of gas particles due to their random motion. Graham‘s Law of Diffusion states that the rate of diffusion of a gas is inversely proportional to the square root of its molar mass.
Mathematically, Graham‘s Law can be expressed as:
Rate of Diffusion ∝ 1/√(Molar Mass)
This means that lighter gases diffuse more rapidly than heavier gases.
The knowledge of gas laws and concepts related to gases is crucial in understanding the behavior and properties of gases, as well as their applications in various fields of science and technology.
Ideal Gas:
An ideal gas is a hypothetical gas that follows the assumptions of the kinetic molecular theory under all conditions of temperature and pressure. The key characteristics of an ideal gas are:
According to the ideal gas law (PV = nRT), an ideal gas obeys the relationship between pressure (P), volume (V), temperature (T), and the number of moles (n) of the gas, where R is the ideal gas constant.
While no real gas perfectly follows all the assumptions of an ideal gas, many gases at low pressures and high temperatures behave closely to the predictions of the ideal gas law.
Real Gas:
A real gas is a gas that does not strictly obey all the assumptions of the kinetic molecular theory, deviating from ideal behavior under certain conditions. Real gases exhibit non-zero volume and experience intermolecular forces, which can affect their behavior.
At high pressures, the volume occupied by gas particles becomes significant, and intermolecular forces become more pronounced, leading to deviations from ideal gas behavior. Real gases also show deviations at low temperatures when the kinetic energy of gas particles is reduced, and intermolecular attractions become more significant.
Real gases can be described using various equations of state, such as the van der Waals equation, which includes corrections for the volume and intermolecular forces.
It‘s important to note that the behavior of a real gas depends on the specific gas and the conditions of temperature and pressure. Different gases may deviate from ideal behavior to varying extents.
The study of ideal and real gases helps in understanding the limitations and deviations from ideal gas behavior, allowing for more accurate predictions and descriptions of gas properties in various applications.
Universal Gas Constant:
The universal gas constant, denoted by R, is a fundamental constant in chemistry and physics that relates the properties of gases to each other. It appears in the ideal gas law, which describes the behavior of ideal gases. The value of the universal gas constant depends on the units used for pressure, volume, temperature, and the amount of gas.
The universal gas constant can be defined using different units:
Significance of the Universal Gas Constant:
The universal gas constant is significant for several reasons:
Overall, the universal gas constant is a fundamental constant that plays a crucial role in understanding and describing the behavior of gases. It allows for the quantitative study of gas properties, conversions between units, and the comparison of gases under different conditions.
Liquid State: Physical Properties of Liquids
Liquids are one of the three states of matter, along with solids and gases. They possess unique physical properties that distinguish them from solids and gases. Some important physical properties of liquids include:
Liquid Crystals and Their Applications
Liquid crystals are a unique phase of matter that exhibits properties of both liquids and solids. They possess an ordered molecular arrangement similar to solids, while still maintaining some degree of fluidity like liquids. Liquid crystals have applications in various fields, including:
Liquid crystals have revolutionized various industries and continue to drive advancements in technology, optics, sensing, and other fields. Their combination of ordered structure and fluidity makes them valuable for a wide range of applications.
Solid State: Introduction and Types of Solids
Solid state is one of the three fundamental states of matter, characterized by a definite shape and volume. Solids are composed of closely packed particles arranged in a regular pattern. Here are some important aspects of the solid state:
The study of the solid state is crucial in understanding the properties and behavior of various materials. It has applications in fields such as materials science, chemistry, geology, and engineering.
Reversible and Irreversible Processes
In thermodynamics, processes are classified as reversible or irreversible based on their ability to be reversed without causing any changes to the surroundings. Let‘s understand these concepts:
It‘s important to note that reversible and irreversible processes are idealized concepts used to simplify the analysis of thermodynamic systems. In reality, most processes lie somewhere between these two extremes.
The distinction between reversible and irreversible processes is crucial in understanding the directionality of energy transfer, the efficiency of energy conversion, and the overall behavior of thermodynamic systems. Engineers and scientists consider the reversibility of processes when designing efficient systems and optimizing energy utilization.
Chemical and Physical Equilibrium
Chemical and physical equilibrium are fundamental concepts in thermodynamics and describe the balance between opposing processes in a system. Let‘s explore these concepts:
In both chemical and physical equilibrium, the system reaches a state of balance where the forward and reverse processes occur simultaneously. Equilibrium is dynamic, meaning that reactions and interconversions continue to take place, but at equal rates. The concept of equilibrium is crucial in understanding reaction kinetics, phase transitions, and the behavior of complex systems.
Chemical equilibrium is often described by the principles of chemical thermodynamics, while physical equilibrium is associated with phase transitions and the properties of matter.
Law of Mass Action:
The law of mass action is a fundamental principle in chemical equilibrium that relates the concentrations of reactants and products in a reversible chemical reaction. It states that the rate of a chemical reaction is directly proportional to the product of the concentrations of the reactants, each raised to the power of its stoichiometric coefficient in the balanced chemical equation.
The general form of the law of mass action for a reversible reaction can be expressed as:
aA + bB ⇌ cC + dD
where A, B, C, and D represent the reactants and products, and a, b, c, and d represent their respective stoichiometric coefficients.
The rate of the forward reaction is given by:
Rate(forward) = kf[A]a[B]b
The rate of the reverse reaction is given by:
Rate(reverse) = kr[C]c[D]d
At equilibrium, the rates of the forward and reverse reactions are equal, resulting in no net change in the concentrations of reactants and products over time.
The equilibrium constant (K) is defined as the ratio of the product of the equilibrium concentrations of the products to the product of the equilibrium concentrations of the reactants, each raised to the power of its stoichiometric coefficient:
K = ([C]c[D]d) / ([A]a[B]b)
The value of the equilibrium constant depends only on the temperature and is a characteristic of the specific chemical reaction. It provides information about the extent of the reaction and the relative concentrations of reactants and products at equilibrium.
Equilibrium Law:
The equilibrium law is a mathematical expression derived from the law of mass action and provides a quantitative relationship between the concentrations of reactants and products at equilibrium. It is used to calculate the equilibrium constant (K) for a given chemical reaction.
The equilibrium law expression is written using the molar concentrations of reactants and products raised to the power of their respective stoichiometric coefficients:
K = ([C]c[D]d) / ([A]a[B]b)
The equilibrium constant is a dimensionless quantity that remains constant at a given temperature, regardless of the initial concentrations of the reactants and products.
The equilibrium constant can provide valuable information about the position of equilibrium. If K > 1, the equilibrium lies towards the products, indicating a higher concentration of products at equilibrium. If K < 1, the equilibrium lies towards the reactants, indicating a higher concentration of reactants at equilibrium. When K=1, the concentrations of reactants and products are approximately equal at equilibrium.
The equilibrium constant is temperature-dependent, and changes in temperature can affect the value of K for a reaction.
Kcand Kpand their Relationship:
In chemical equilibrium, the equilibrium constant (K) can be expressed in terms of either concentrations (Kc) or partial pressures (Kp), depending on the nature of the reaction and the units used for expressing concentrations or pressures.
Kcis the equilibrium constant expressed in terms of molar concentrations of the reactants and products. It is determined by writing the balanced chemical equation and expressing the concentrations of the species involved in the reaction. The equilibrium constant equation in terms of concentrations is:
Kc= ([C]c[D]d) / ([A]a[B]b)
On the other hand, Kpis the equilibrium constant expressed in terms of partial pressures of the reactants and products. It is commonly used for reactions involving gases. The equilibrium constant equation in terms of partial pressures is obtained by using the ideal gas law and expressing the partial pressures of the species involved:
Kp= (PCc* PDd) / (PAa* PBb)
where PA, PB, PC, and PDare the partial pressures of the reactants and products.
The relationship between Kcand Kpdepends on the stoichiometry of the balanced chemical equation. For a balanced chemical equation of the form:
aA + bB ⇌ cC + dD
The relationship between Kpand Kcis given by:
Kp= Kc* (RT)Δn
where R is the gas constant, T is the temperature in Kelvin, and Δn is the difference between the sum of the coefficients of the product species and the sum of the coefficients of the reactant species.
If Δn is positive, Kpwill be greater than Kc, indicating a higher preference for the products in terms of partial pressures. If Δn is negative, Kpwill be less than Kc, indicating a higher preference for the reactants in terms of partial pressures.
It is important to note that the relationship between Kcand Kpholds true only for reactions involving gases and when the partial pressures are used as the concentrations of the gases.
Effect of Change of External Conditions in Dynamic Equilibrium:
In a dynamic equilibrium, the forward and reverse reactions are occurring at the same rate, resulting in a constant concentration of reactants and products. However, the equilibrium can be disturbed by changing external conditions such as temperature, pressure, or concentration. The Le Chatelier‘s principle describes the effect of these changes on the equilibrium position.
1. Effect of Temperature:
Changing the temperature can shift the equilibrium position. According to Le Chatelier‘s principle:
2. Effect of Pressure (for Gas Reactions):
For reactions involving gases, changing the pressure can affect the equilibrium position. According to Le Chatelier‘s principle:
3. Effect of Concentration:
Changing the concentration of reactants or products can also impact the equilibrium position:
Conversely, if the concentration of reactants or products is decreased, the equilibrium will shift in the opposite direction to replenish the decrease.
It is important to note that the changes in external conditions do not disrupt the equilibrium itself, but rather cause a temporary shift in the equilibrium position until a new equilibrium is established to counteract the change.
Types of Hydrogen:
Hydrogen exists in three isotopic forms: protium, deuterium, and tritium. These isotopes have different numbers of neutrons in their nuclei, resulting in variations in their atomic mass. The three types of hydrogen are:
| Hydrogen Isotope | Number of Protons | Number of Neutrons | Atomic Mass |
|---|---|---|---|
| Protium | 1 | 0 | 1.007825 amu |
| Deuterium | 1 | 1 | 2.014102 amu |
| Tritium | 1 | 2 | 3.016049 amu |
Protium is the most abundant and commonly found isotope of hydrogen, while deuterium and tritium are relatively rare.
Isotopes of Hydrogen:
Isotopes are variants of an element that have the same number of protons but different numbers of neutrons. The isotopes of hydrogen are:
Deuterium and tritium are used in various scientific and industrial applications.
Applications of Hydrogen:
Hydrogen has several important applications across various fields. Some of the notable applications of hydrogen include:
These are just a few examples of the numerous applications of hydrogen in different sectors, highlighting its versatility and importance in various industries.
Heavy Water:
Heavy water is a form of water in which the hydrogen atoms are replaced with deuterium, an isotope of hydrogen with one proton and one neutron. It is represented by the chemical formula D₂O or ^2H₂O. In heavy water, the oxygen atom remains the same as in regular water (H₂O).
Heavy water has unique properties compared to regular water due to the presence of deuterium. Some important features of heavy water include:
Heavy water finds applications in various fields, including:
It‘s important to note that heavy water is relatively rare and more expensive to produce compared to regular water. Its specialized properties make it valuable in specific applications, particularly in nuclear-related industries and scientific research.
Oxygen:
Oxygen is a chemical element with the symbol O and atomic number 8. It is a highly reactive nonmetal and is essential for the support of life on Earth. Oxygen makes up about 21% of the Earth‘s atmosphere and is the third-most abundant element in the universe.
Allotropy of Oxygen:
Oxygen exhibits two primary allotropes, namely oxygen (O₂) and ozone (O₃):
Oxides:
Oxygen combines with various elements to form oxides. Oxides are compounds that contain oxygen bonded to one or more other elements. Some examples of oxides include carbon dioxide (CO₂), water (H₂O), and iron oxide (Fe₂O₃). Oxides have diverse properties and play important roles in chemical reactions, materials, and environmental processes.
Applications of Hydrogen Peroxide:
Hydrogen peroxide (H₂O₂) is a compound composed of hydrogen and oxygen. It has several applications, including:
Medical and Industrial Applications of Oxygen:
Oxygen has a wide range of applications in both medical and industrial sectors:
Oxygen is a versatile element with vital roles in numerous fields, ranging from sustaining life to industrial applications. Its unique properties and reactivity make it indispensable in various scientific, medical, and industrial endeavors.
Ozone:
Ozone is a molecule composed of three oxygen atoms, represented as O₃. It is a pale blue gas with a distinct odor and is chemically reactive. Ozone plays a crucial role in both the Earth‘s atmosphere and various applications.
Occurrence:
Ozone occurs naturally in the Earth‘s atmosphere, particularly in the ozone layer located in the stratosphere. It forms when oxygen molecules (O₂) are exposed to ultraviolet (UV) radiation from the Sun, causing them to dissociate into individual oxygen atoms. These oxygen atoms can then react with other oxygen molecules to form ozone.
Preparation of Ozone:
Ozone can be prepared through various methods, including:
Structure:
Ozone has a bent molecular structure, with an angle of approximately 117 degrees between the oxygen atoms. It forms a resonance structure due to the delocalization of the unpaired electron, resulting in its high reactivity.
Test for Ozone:
Ozone can be detected using various tests, including:
Ozone Layer Depletion:
Ozone layer depletion refers to the thinning of the ozone layer in the stratosphere due to the release of certain chemicals, such as chlorofluorocarbons (CFCs) and halons. These substances, primarily used in aerosols, refrigerants, and solvents, can reach the stratosphere and break down ozone molecules through catalytic reactions. Ozone layer depletion poses a threat to the Earth‘s ecosystems and increases the risk of harmful UV radiation reaching the surface.
Applications of Ozone:
Ozone has several applications in various fields:
Ozone‘s unique properties and reactivity make it a valuable substance in different applications. However, its presence in the Earth‘s atmosphere must be carefully regulated to maintain the balance of the ozone layer and protect the environment.
Nitrogen:
Nitrogen is an essential element that is widely present in the Earth‘s atmosphere. It is a non-metal and forms the majority of the air we breathe. Nitrogen plays a vital role in various biological and industrial processes.
Inertness of Nitrogen Molecule:
The nitrogen molecule (N₂) is highly stable and relatively unreactive due to the triple bond between the nitrogen atoms. This inertness makes nitrogen gas useful for applications requiring an inert atmosphere, such as in food packaging, chemical synthesis, and electronic manufacturing.
Ammonia:
Ammonia (NH₃) is a compound composed of nitrogen and hydrogen. It is a colorless gas with a pungent odor. Ammonia is widely used in the production of fertilizers, cleaning agents, and various industrial processes. It is also a vital source of nitrogen for plants in agriculture.
Applications of Ammonia:
Ammonia finds applications in various sectors:
Harmful Effects of Ammonia:
Although ammonia has various industrial applications, it can be harmful to human health. Ammonia is corrosive and can cause irritation to the eyes, skin, and respiratory system. Inhalation of high concentrations of ammonia vapor can be toxic and may lead to severe respiratory distress.
Oxyacids of Nitrogen and Nitric Acid:
Nitrogen forms several oxyacids, with nitric acid (HNO₃) being the most well-known. Nitric acid is a strong acid and a powerful oxidizing agent. It is widely used in the production of fertilizers, explosives, dyes, and various other chemicals.
Chemical Properties:
Nitrogen exhibits various chemical properties, including its ability to form covalent bonds with other elements, its involvement in oxidation-reduction reactions, and its role in the nitrogen cycle, which is crucial for maintaining the balance of nitrogen in ecosystems.
Ring Test for Nitrate:
The ring test is a qualitative test used to detect the presence of nitrates. In this test, a sample suspected to contain nitrates is mixed with concentrated sulfuric acid and heated. The formation of a brown ring at the junction of the two layers indicates the presence of nitrates.
Halogens:
Halogens are a group of highly reactive non-metallic elements found in Group 17 of the periodic table. The group includes fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At). They exhibit similar chemical properties due to their outermost electron configuration and are known for their high reactivity and ability to form salts.
General Characteristics of Halogens:
Preparation of Halogens:
The halogens can be prepared through various methods:
| Halogen | Preparation Method |
|---|---|
| Fluorine (F₂) | Electrolysis of a solution containing fluoride ions. |
| Chlorine (Cl₂) | Electrolysis of a solution containing chloride ions (chloralkali process) or by the reaction of hydrochloric acid with an oxidizing agent. |
| Bromine (Br₂) | By the oxidation of bromide ions using an oxidizing agent or by the reaction of bromides with chlorine. |
| Iodine (I₂) | By the reaction of iodides with an oxidizing agent, such as concentrated sulfuric acid. |
Tests of Halogens:
Halogens can be identified using various tests:
Halo Acids:
Halo acids are acids in which one or more hydrogen atoms of the acid are replaced by halogen atoms. The most common examples are hydrochloric acid (HCl), hydrobromic acid (HBr), and hydroiodic acid (HI). These acids are highly soluble in water and are strong acids.
The chemical equations for the preparation and tests of halogens are as follows:
2NaCl + 2H₂O →2NaOH + Cl₂↑ + H₂↑
2KBr + Cl₂ →2KCl + Br₂↑
2NaI + 2H₂SO₄ + H₂O₂ →I₂↑ + 2NaHSO₄ + 2H₂O
Cl₂ + 2NaOH →NaCl + NaClO + H₂O
Br₂ + 2NaOH →NaBr + NaBrO + H₂O
I₂ + 2NaOH →NaI + NaIO + H₂O
Carbon:
Carbon is a chemical element with the symbol C and atomic number 6. It is a nonmetal and the basis of organic chemistry due to its ability to form a wide variety of compounds. Carbon is the fourth most abundant element in the universe and plays a crucial role in the chemistry of life.
Allotropy of Carbon:
Carbon exhibits allotropy, which means it can exist in different physical forms with varying structures and properties. The main allotropes of carbon are:
Allotropic Modifications of Carbon:
| Allotrope | Structure | Properties |
|---|---|---|
| Diamond | Three-dimensional crystal lattice | Hard, transparent, high melting point |
| Graphite | Layered structure | Soft, good conductor of electricity, lubricating |
| Fullerenes | Closed-cage or hollow structures | Unique electrical and physical properties |
| Amorphous Carbon | No defined crystalline structure | Variable properties depending on the type |
Carbon Monoxide:
Carbon monoxide (CO) is a colorless, odorless gas formed by the incomplete combustion of carbon-containing materials. It is highly toxic and can be produced from sources such as car exhaust, gas heaters, and faulty appliances. Carbon monoxide binds strongly to hemoglobin in the blood, reducing its oxygen-carrying capacity and posing a severe health risk.
Phosphorus:
Phosphorus is a chemical element with the symbol P and atomic number 15. It is a nonmetal that plays a crucial role in various biological processes and is essential for life. Phosphorus compounds are widely used in fertilizers, detergents, and other industrial applications.
Allotropic Forms of Phosphorus:
Phosphorus exhibits several allotropic forms, including:
Phosphine (PH3):
Phosphine, also known as phosphorus trihydride, is a colorless and highly toxic gas with the chemical formula PH3. It is produced by the reaction of a phosphorus source with a reducing agent, such as calcium phosphide. Phosphine has a pungent odor and is flammable. It is used in various applications, including as a fumigant for stored grain and as a reducing agent in organic synthesis.
Sulfur:
Sulfur is a chemical element with the symbol S and atomic number 16. It is a nonmetal that is commonly found in nature and has various industrial applications. Sulfur is known for its yellow color and distinctive odor.
Allotropy of Sulfur:
Sulfur exhibits several allotropes, including:
Hydrogen Sulphide (H2S):
Hydrogen sulphide is a colorless and highly toxic gas with the chemical formula H2S. It has a characteristic rotten egg odor and is commonly associated with the smell of sulfur. Hydrogen sulphide is produced by the decay of organic matter and is found in natural gas, volcanic gases, and some mineral springs.
Sulfur Dioxide (SO2):
Sulfur dioxide is a colorless gas with a pungent odor. It has the chemical formula SO2 and is produced by the combustion of sulfur-containing fuels, such as coal and oil, as well as by volcanic activity. Sulfur dioxide is a major air pollutant and is also used as a preservative in food and wine.
Properties of H2SO4 (Sulfuric Acid):
Sulfuric acid, commonly known as battery acid, is a strong and highly corrosive acid with the chemical formula H2SO4. It is a dense, oily liquid that is soluble in water and has a wide range of industrial applications. Some properties of sulfuric acid include:
Sodium Thiosulphate:
Sodium thiosulphate, also known as sodium thiosulfate, is a white crystalline compound with the chemical formula Na2S2O3. It is used in various applications, including photography as a fixing agent to remove unexposed silver halides from photographic films and papers. Sodium thiosulphate is also used as an antidote for cyanide poisoning.
Metals:
Metals are a group of elements that are characterized by their shiny appearance, good conductance of heat and electricity, malleability (ability to be hammered into thin sheets), and ductility (ability to be drawn into wires). They are typically solid at room temperature (except for mercury) and have high melting and boiling points. Metals are found on the left side of the periodic table, and they tend to lose electrons to form positive ions (cations) in chemical reactions.
Metalloids:
Metalloids, also known as semi-metals, are elements that exhibit properties of both metals and non-metals. They are located along the "staircase" on the periodic table, separating metals from non-metals. Metalloids have intermediate conductivity, varying degrees of luster, and can behave as either electron donors or acceptors in chemical reactions.
Non-metals:
Non-metals are a group of elements that are generally poor conductors of heat and electricity, have low melting and boiling points, and exhibit diverse physical properties. Non-metals are found on the upper right side of the periodic table, and they tend to gain electrons to form negative ions (anions) in chemical reactions. Non-metals can exist in various states of matter, including solids (such as sulfur and iodine), liquids (such as bromine), and gases (such as oxygen and nitrogen).
These three categories of elements—metals, metalloids, and non-metals—help classify and differentiate the properties and behaviors of various elements in the periodic table.
Ores:
Ores are naturally occurring rocks or minerals from which metals or valuable substances can be extracted economically. They serve as the primary source of metals and are obtained through mining and extraction processes. Ores can be found in various geological formations and can contain a wide range of elements, including metals like iron, copper, gold, silver, aluminum, and many others.
The extraction of metals from ores involves various methods such as crushing, grinding, heating, and chemical reactions to separate the desired metal from the ore. Ores are typically processed to obtain pure metals or compounds that can be further used for industrial purposes, manufacturing, and construction.
Gangue or Matrix:
Gangue or matrix refers to the unwanted or non-valuable materials that are present in ores. These materials are usually impurities or compounds that do not contain the desired metal or have low economic value. Gangue minerals can vary depending on the type of ore and the specific geological conditions where it is found.
During the extraction process, the gangue is separated from the ore to obtain a concentrated form of the desired metal or mineral. Various techniques such as froth flotation, magnetic separation, and smelting are employed to remove the gangue and isolate the valuable components of the ore.
The presence of gangue in ores poses challenges in terms of extraction efficiency and cost. Minimizing the amount of gangue in the final product is important to ensure high purity and quality of the extracted metal or mineral.
Alloy:
An alloy is a homogeneous mixture or solid solution composed of two or more metals, or a metal and a non-metal. Alloys are formed by combining different elements to enhance the properties of the resulting material, such as strength, durability, resistance to corrosion, and electrical conductivity. The process of alloying involves melting the metals or elements together and then allowing them to solidify.
Alloys can have a wide range of compositions and can be classified into different categories based on their composition and properties. Some common examples of alloys include bronze (copper and tin), brass (copper and zinc), stainless steel (iron, chromium, and nickel), and bronze (copper and aluminum).
Alloys have numerous applications in various industries, including construction, automotive, aerospace, electronics, and jewelry. They offer improved properties and versatility compared to pure metals, making them suitable for specific purposes and requirements.
Amalgam:
Amalgam is a type of alloy that specifically refers to the combination of a metal with mercury. The most well-known example of amalgam is dental amalgam, which is used for filling cavities in teeth. Dental amalgam typically consists of a mixture of silver, tin, copper, and mercury.
Amalgamation is a process that involves dissolving a metal in mercury to form a homogeneous mixture. The mercury acts as a binding agent, allowing the metal particles to be easily shaped and manipulated. Amalgams have the unique property of being soft and pliable when first mixed but hardening over time to form a solid material.
In addition to dental applications, amalgams have been used historically in various other areas, such as silvering mirrors, gold extraction in mining, and electrical applications. However, the use of mercury in amalgams has raised environmental and health concerns, leading to restrictions and the development of alternative materials.
Metallurgy:
Metallurgy is the branch of science and technology that deals with the extraction, production, purification, and study of metals and their alloys. It involves various processes and techniques to obtain metals from their ores, purify them, and transform them into useful materials for industrial applications.
Metallurgy plays a crucial role in the development of modern society as metals are essential for infrastructure, transportation, electronics, manufacturing, and many other industries. It encompasses a wide range of processes, from mining and ore processing to metal refining and fabrication.
Types or Branches of Metallurgy:
1.Extractive Metallurgy:It focuses on the extraction of metals from their ores and the production of crude metal. This branch involves processes such as mining, crushing, grinding, concentration, roasting, smelting, and refining.
2.Physical Metallurgy:It deals with the physical and mechanical properties of metals and their alloys. This branch includes the study of crystal structure, phase transformations, heat treatment, mechanical behavior, and metallography.
3.Chemical Metallurgy:It involves the chemical aspects of metallurgy, such as the development of chemical processes for metal extraction, refining, and purification. This branch focuses on the use of chemical reactions and principles in metallurgical processes.
4.Mechanical Metallurgy:It deals with the mechanical properties and behavior of metals and alloys, including their strength, ductility, toughness, and fatigue resistance. This branch includes the study of deformation mechanisms, mechanical testing, and the design of metal components.
5.Industrial Metallurgy:It refers to the application of metallurgical principles and techniques in large-scale industrial processes. This branch focuses on the efficient and economical production of metals and alloys for commercial purposes.
These branches of metallurgy are interconnected and often overlap in their applications and research areas. They collectively contribute to the understanding, advancement, and utilization of metals in various industries.
General Principles of Extraction of Metals:
The extraction of metals from their ores involves various processes and techniques. Here are the general principles followed in the extraction of metals:
1.Crushing and Grinding:The ore is first crushed and ground into fine particles to increase the surface area for chemical reactions.
2.Concentration of Ore:The process of removing impurities from the ore to obtain a concentrated form is called ore concentration or beneficiation. It is done through various methods such as gravity separation, magnetic separation, froth flotation, etc.
3.Roasting and Calcination:Some ores require heating in the presence of air (roasting) or without air (calcination) to convert them into more desirable forms for further processing.
4.Reduction:The process of extracting metals from their oxides or other compounds by chemical reduction is called reduction. This is usually done by heating the ore with a reducing agent such as carbon or hydrogen.
5.Electrolytic Reduction:Some metals, such as aluminum and sodium, are extracted through electrolysis. In this process, the metal ions are reduced at the cathode electrode using electrical energy.
6.Refining:The extracted metal is often impure and requires further purification. Refining methods such as electrolytic refining, zone refining, and distillation are used to remove impurities and obtain a pure metal.
7.Alloying:Metals are often mixed with other elements to improve their properties. Alloying is the process of combining metals with one or more other elements to form alloys.
8.Recycling:Recycling of metals is an important aspect of sustainable metal extraction. It involves collecting and processing scrap metals to recover valuable metals for reuse.
These general principles form the basis for extracting various metals from their ores. The specific extraction methods and techniques may vary depending on the type of metal and the nature of the ore.
Purification or Refining of Metals:
After the extraction of metals from their ores, they often need to undergo purification or refining processes to remove impurities and obtain a pure metal. Here are some common methods used for the purification of metals:
1.Electrolytic Refining:This method is used for the purification of metals that can be obtained through electrolysis. The impure metal is made the anode, and a pure metal is used as the cathode. When an electric current is passed through the electrolyte, the metal ions from the anode migrate to the cathode and get deposited, resulting in the purification of the metal.
2.Zinc-Hg Amalgamation:This method is used for the purification of metals like gold and silver. The impure metal is mixed with mercury (Hg) to form an amalgam. The amalgam is then heated, and the volatile mercury evaporates, leaving behind the purified metal.
3.Distillation:Distillation is used for the purification of metals that have low boiling points. The impure metal is heated to vaporize it, and then the vapor is collected and condensed to obtain the pure metal.
4.Zone Refining:This method is used for the purification of metals with high melting points and low solubility in the molten state. The impure metal is melted, and a small molten zone is passed through it. As the zone moves, impurities get concentrated in the molten region and are removed. This process is repeated several times to obtain a highly purified metal.
5.Chromatographic Methods:Chromatographic methods, such as column chromatography and paper chromatography, are used for the purification of certain metals. The impure metal is dissolved in a suitable solvent and passed through a column or a paper strip. Different components of the metal mixture move at different rates, allowing for their separation.
6.Crystallization:Crystallization is a purification method used for metals that form crystals. The impure metal is dissolved in a suitable solvent, and the solution is allowed to cool slowly. As the solution cools, pure metal crystals start forming, while impurities remain in the solution or form separate crystals that can be separated.
These are some of the common methods used for the purification or refining of metals. The choice of method depends on the specific metal and its impurities. The goal is to remove impurities and obtain a high-purity metal suitable for various applications.
Alkali Metals:
Alkali metals are a group of elements located in Group 1 of the periodic table. They include lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr). Here are some general characteristics of alkali metals:
1.Metallic Properties:Alkali metals are highly reactive metals with shiny appearances. They have low densities and low melting and boiling points.
2.Softness:Alkali metals are very soft and can be easily cut with a knife. This is due to their low cohesive forces between atoms.
3.Low Ionization Energies:Alkali metals have low ionization energies, meaning it requires relatively little energy to remove an electron from an alkali metal atom. As a result, they readily lose their outermost electron to form positive ions.
4.Reactivity:Alkali metals are highly reactive, especially with water and oxygen. They react vigorously with water, releasing hydrogen gas and forming metal hydroxides. They also react with oxygen in the air, forming oxides.
Now, let‘s take a closer look at sodium, one of the alkali metals, and two of its important compounds:
Sodium:
- Sodium (Na) is a silver-white metal that is highly reactive.
Sodium Hydroxide:
- Sodium hydroxide (NaOH), also known as caustic soda, is a strong base. It is produced through the electrolysis of sodium chloride (NaCl) solution in a process called thechloralkali process. Sodium hydroxide is widely used in industries such as chemical manufacturing, pulp and paper production, and soap making.
Sodium Carbonate:
- Sodium carbonate (Na2CO3), also known as soda ash or washing soda, is a white crystalline solid. It is commonly used in the production of glass, detergents, and chemicals. Sodium carbonate can be obtained from naturally occurring deposits or synthesized through the Solvay process.
These are some of the key aspects of alkali metals, with a focus on sodium and two of its important compounds: sodium hydroxide and sodium carbonate.
Alkaline Earth Metals:
Alkaline earth metals are a group of elements located in Group 2 of the periodic table. They include beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra). Here are some general characteristics of alkaline earth metals:
1.Metallic Properties:Alkaline earth metals are shiny, silvery-white metals with relatively low densities and melting points compared to other metals.
2.Reactivity:Alkaline earth metals are less reactive than alkali metals but still more reactive than most other metals. They readily lose their two outermost electrons to form divalent cations with a +2 charge.
3.Higher Ionization Energies:Alkaline earth metals have higher ionization energies compared to alkali metals, meaning more energy is required to remove their electrons.
4.Useful Compounds:Alkaline earth metals form various useful compounds, including oxides, hydroxides, carbonates, sulfates, and nitrates. These compounds have applications in industries such as construction, agriculture, and medicine.
Now, let‘s explore some specific characteristics and compounds of alkaline earth metals:
Solubility of Alkaline Earth Metal Hydroxides, Carbonates, and Sulfates:
- Alkaline earth metal hydroxides (e.g., magnesium hydroxide, calcium hydroxide) are sparingly soluble in water, forming alkaline solutions. The solubility generally increases as you move down the group.
- Alkaline earth metal carbonates (e.g., magnesium carbonate, calcium carbonate) are insoluble or sparingly soluble in water. However, they can react with acids to produce carbon dioxide gas.
- Alkaline earth metal sulfates (e.g., magnesium sulfate, calcium sulfate) are generally soluble in water. However, the solubility decreases as you move down the group.
Thermal Decomposition of Alkaline Earth Metal Carbonates and Nitrates:
- Alkaline earth metal carbonates undergo thermal decomposition when heated, producing metal oxides and carbon dioxide. The thermal stability generally increases as you move down the group.
- Alkaline earth metal nitrates also undergo thermal decomposition when heated, releasing oxygen gas and leaving behind metal oxides and nitrogen dioxide gas.
These are some of the general characteristics and specific features of alkaline earth metals, including the solubility patterns of their hydroxides, carbonates, and sulfates, as well as the thermal decomposition of their carbonates and nitrates.
Bio - Inorganic Chemistry:
Bio-inorganic chemistry is the field of study that explores the role of inorganic elements and compounds in biological systems. It focuses on the interactions between inorganic elements and biological molecules, as well as their functions and significance in living organisms. Inorganic elements play crucial roles in various biological processes, such as enzyme catalysis, electron transport, oxygen transport, and structural support.
Elements in the human body:
The human body consists of a diverse range of elements, both inorganic and organic, which are essential for maintaining various physiological functions. Here are some of the key inorganic elements found in the human body:
| Element | Function |
|---|---|
| Calcium (Ca) | Important for bone and teeth formation, muscle contraction, and nerve function. |
| Iron (Fe) | Essential component of hemoglobin in red blood cells, involved in oxygen transport. |
| Sodium (Na) | Plays a role in nerve impulse transmission, muscle contraction, and fluid balance. |
| Potassium (K) | Important for nerve function, muscle contraction, and maintaining fluid balance. |
| Zinc (Zn) | Required for enzyme catalysis, gene expression, and immune system function. |
| Copper (Cu) | Involved in electron transport, enzyme activation, and connective tissue formation. |
| Magnesium (Mg) | Plays a role in enzyme catalysis, energy metabolism, and muscle function. |
| Chlorine (Cl) | Essential for maintaining fluid balance, acid-base balance, and nerve function. |
These are just a few examples of inorganic elements found in the human body. Each element serves a specific function and is vital for overall health and well-being.
Bio-inorganic chemistry plays a crucial role in understanding the interactions between these inorganic elements and biological systems. It helps uncover the mechanisms by which these elements contribute to various physiological processes, as well as the impact of their deficiency or excess on human health.
Macro and Micronutrients:
Nutrients are substances that are essential for the growth, development, and maintenance of living organisms. They provide energy, support metabolic processes, and contribute to overall health and well-being. Nutrients are broadly classified into two categories: macro and micronutrients, based on the quantity required by the body.
Macro Nutrients:
Macro nutrients are nutrients that are required by the body in relatively large amounts. They provide energy and are necessary for the proper functioning of the body. The three main macro nutrients are:
Micronutrients:
Micronutrients are nutrients that are required by the body in smaller amounts but are still essential for proper functioning. They include vitamins and minerals. Here are some examples of micronutrients:
The body needs both macro and micronutrients in the right balance to maintain optimal health. While macro nutrients provide the bulk of energy, micronutrients are involved in crucial biochemical reactions and contribute to overall well-being.
A balanced diet that includes a variety of foods from different food groups is essential to ensure an adequate intake of both macro and micronutrients.
Importance of Metal Ions in Biological Systems:
Metal ions play vital roles in various biological systems and are essential for the proper functioning of living organisms. They are involved in a wide range of biochemical processes and are required in trace amounts for important biological functions. Here are some key reasons for the importance of metal ions in biological systems:
These are just a few examples of the diverse roles of metal ions in biological systems. Their presence and proper regulation are crucial for various physiological processes, and any imbalance or deficiency can have significant impacts on health and well-being.
Active Transport and Cellular Ion Pump:
Active transport is a vital process in cells that allows the movement of ions or molecules against their concentration gradient. It requires the expenditure of energy to transport substances from an area of lower concentration to an area of higher concentration. One important mechanism of active transport is through cellular ion pumps. Two well-known examples of cellular ion pumps are the Na-K pump (primary active transport) and the sodium-glucose pump (secondary active transport).
1. Na-K Pump (Sodium-Potassium Pump):
The Na-K pump is a primary active transport system found in the cell membrane of most animal cells. It utilizes ATP (adenosine triphosphate) as a source of energy to transport sodium (Na+) and potassium (K+) ions across the cell membrane against their concentration gradients.
The process involves the following steps:
The Na-K pump maintains the concentration gradients of Na+ and K+ across the cell membrane, with higher Na+ concentration outside the cell and higher K+ concentration inside the cell. This gradient is important for various cellular processes, including nerve impulse transmission and muscle contraction.
2. Sodium-Glucose Pump (Secondary Active Transport):
The sodium-glucose pump is an example of secondary active transport that couples the movement of sodium ions (Na+) and glucose molecules across the cell membrane. It relies on the electrochemical gradient created by the Na-K pump.
The process involves the following steps:
This process allows the uptake of glucose into cells, such as in the intestinal lining for nutrient absorption or in kidney tubules for glucose reabsorption.
Both primary and secondary active transport mechanisms play essential roles in maintaining ion concentrations and nutrient uptake in cells, contributing to various physiological processes and cellular functions.
Heavy Metal Toxicity:
Heavy metals are metallic elements with high atomic weights that can have toxic effects on living organisms. These metals include lead, mercury, cadmium, arsenic, and many others. Heavy metal toxicity occurs when these metals accumulate in the body at high levels, leading to harmful effects on health.
Sources of Heavy Metal Exposure:
Heavy metals can enter the environment through natural processes, such as volcanic activity and weathering of rocks, as well as through human activities. Some common sources of heavy metal exposure include:
Effects of Heavy Metal Toxicity:
Heavy metals can exert toxic effects on various organs and systems in the body. The specific effects depend on the type of metal, duration of exposure, and individual susceptibility. Some common effects of heavy metal toxicity include:
Prevention and Treatment:
Preventing heavy metal toxicity involves minimizing exposure to these metals. This can be achieved through:
If heavy metal toxicity is suspected, medical intervention is necessary. Treatment may involve:
It is essential to raise awareness about heavy metal toxicity and implement measures to reduce exposure and protect human health and the environment.
Introduction to Organic Chemistry:
Organic chemistry is the branch of chemistry that deals with the study of organic compounds. Organic compounds are compounds primarily composed of carbon atoms bonded with other elements such as hydrogen, oxygen, nitrogen, sulfur, and halogens. Carbon is a unique element that has the ability to form a wide variety of compounds due to its ability to form stable covalent bonds with other atoms.
Organic chemistry plays a crucial role in various fields, including pharmaceuticals, materials science, biochemistry, agriculture, and environmental science. It explores the structure, properties, composition, reactions, and synthesis of organic compounds.
Organic Compounds:
Organic compounds are diverse and can be found in various forms, ranging from simple hydrocarbons to complex biomolecules. They can be classified into different groups based on their functional groups and structural characteristics. Some common types of organic compounds include:
Organic compounds exhibit a wide range of physical and chemical properties and can participate in various types of reactions, such as combustion, substitution, addition, and polymerization. Understanding organic chemistry is essential for the development of new drugs, materials, and technologies, as well as the study of life processes and environmental interactions.
Tetravalency of Carbon:
Carbon is a unique element due to its tetravalent nature, meaning it has the ability to form four covalent bonds with other atoms. This property is a result of carbon‘s electronic configuration, with four valence electrons in its outermost energy level.
The tetravalent nature of carbon allows it to form stable covalent bonds with a variety of elements, including hydrogen, oxygen, nitrogen, sulfur, and halogens. It can form single, double, and triple bonds depending on the number of electrons it shares with other atoms. Carbon‘s ability to form multiple bonds and its flexibility in bonding arrangements give rise to the vast diversity of organic compounds.
Tetravalency also enables carbon to form long chains, branched structures, and rings, giving rise to the complexity and versatility of organic molecules. These carbon-based structures are the building blocks of life and the foundation of organic chemistry.
Catenation Property of Carbon:
The catenation property of carbon refers to its ability to form covalent bonds with other carbon atoms, resulting in the formation of long chains or networks. This property is unique to carbon and is responsible for the formation of carbon compounds with diverse structures and properties.
Carbon has a strong affinity for forming bonds with other carbon atoms due to its small atomic size and the strength of carbon-carbon bonds. This allows carbon to form stable and extended chains, which can be straight, branched, or cyclic. The catenation property of carbon is the basis for the existence of hydrocarbons and organic molecules with complex structures.
The ability of carbon to form long chains and complex networks contributes to the stability and structural diversity of organic compounds. It allows for the existence of different isomers, where atoms are arranged in different ways while maintaining the same molecular formula. The catenation property of carbon is crucial for the vast array of organic compounds found in nature and the functionality and complexity of organic chemistry.
Classification of Organic Compounds:
Organic compounds are classified based on their functional groups, which are specific arrangements of atoms that determine the chemical properties and reactivity of the compounds. Here are some major classes of organic compounds:
| Class | Functional Group | Examples |
|---|---|---|
| Hydrocarbons | Only carbon and hydrogen atoms | Methane (CH4), Ethane (C2H6), Benzene (C6H6) |
| Alcohols | -OH (hydroxyl) group | Methanol (CH3OH), Ethanol (C2H5OH), Propanol (C3H7OH) |
| Aldehydes | -CHO (carbonyl) group | Methanal (formaldehyde), Ethanal (acetaldehyde), Propanal |
| Ketones | -C=O (carbonyl) group | Propanone (acetone), Butanone (methylethylketone), Hexanone |
| Carboxylic Acids | -COOH (carboxyl) group | Methanoic acid (formic acid), Ethanoic acid (acetic acid), Propanoic acid |
| Esters | -COO- (ester) group | Methyl ethanoate (methyl acetate), Ethyl propanoate, Butyl butanoate |
| Amines | -NH2(amino) group | Methylamine, Ethylamine, Propylamine |
| Amides | -CONH2(amide) group | Acetamide, Formamide, Propionamide |
| Ethers | R-O-R‘ (ether) group | Dimethyl ether, Diethyl ether, Methyl ethyl ether |
These are just a few examples of the many classes of organic compounds. Each class has unique properties and characteristic reactions based on the functional group present. Organic compounds play a vital role in various biological, industrial, and environmental processes, and their classification helps in understanding their behavior and applications.
Alkyl Groups:
An alkyl group is a functional group derived from an alkane by removing one hydrogen atom. It is represented by the general formula CnH2n+1. Alkyl groups are often considered as substituents attached to a parent hydrocarbon chain or molecule, and they significantly influence the properties and reactivity of organic compounds.
Some common alkyl groups include:
Functional Groups:
A functional group is a specific arrangement of atoms within an organic molecule that determines its chemical properties and behavior. Functional groups are responsible for the characteristic reactions and properties of organic compounds. They play a crucial role in determining the reactivity, solubility, and physical properties of organic molecules.
Some common functional groups include:
Homologous Series:
A homologous series is a group of organic compounds that have the same functional group and exhibit a similar chemical behavior. The compounds in a homologous series have a regular gradation in their physical properties, such as molecular weight, boiling point, and chemical reactivity, as the number of carbon atoms in the compound increases.
Each member of a homologous series can be obtained by replacing a hydrogen atom in the previous member with a methyl group (-CH3). The general formula of a homologous series can be represented as follows:
R-CH2-CH2-...-CH2-R
For example, the homologous series of alkanes is represented by the general formula CnH2n+2. Each member of this series, such as methane (CH4), ethane (C2H6), propane (C3H8), follows the same pattern of having one more carbon atom and two more hydrogen atoms than the previous member.
Types of Formulas:
Formulas are used to represent the chemical composition and structure of compounds. In organic chemistry, various types of formulas are employed to describe different aspects of organic compounds. Here are some commonly used types of formulas:
These different types of formulas provide various levels of information about the composition, structure, and connectivity of organic compounds, allowing chemists to communicate and understand the properties and behavior of organic substances.
Cracking:
Cracking is a process used in petroleum refining to break down large hydrocarbon molecules into smaller, more useful molecules. It involves the breaking of carbon-carbon bonds in the long-chain hydrocarbons to produce shorter-chain hydrocarbons. The main purpose of cracking is to increase the yield of gasoline and other valuable products.
There are two primary types of cracking processes:
Cracking processes are vital in the petroleum industry as they help convert heavy, less valuable fractions of crude oil into lighter, more valuable products like gasoline, diesel, and aviation fuel.
Reforming:
Reforming is a process used to convert low-octane naphtha (a petroleum fraction) into high-octane gasoline and other valuable products. It involves the rearrangement of hydrocarbon molecules to produce branched or cyclic structures, which improves the octane rating of the gasoline.
The reforming process primarily consists of two steps:
Reforming is an essential process in the petroleum industry as it improves the quality of gasoline by increasing its octane rating. High-octane gasoline is desirable as it provides better performance and reduces engine knocking.
Both cracking and reforming are crucial processes in the petroleum industry for the production of various valuable products and optimizing the use of petroleum resources.
IUPAC Naming of Organic Compounds:
The IUPAC (International Union of Pure and Applied Chemistry) system provides a standardized method for naming organic compounds based on their structure and functional groups. Here is the general format for IUPAC naming of organic compounds:
Here is an example to illustrate the format:
Compound: 2-methylbut-1-ene
Explanation:
The systematic use of the IUPAC naming system ensures consistent and unambiguous names for organic compounds, allowing for clear communication and understanding in the field of organic chemistry.
Nomenclature of Unsaturated Hydrocarbons:
Unsaturated hydrocarbons are organic compounds that contain double or triple bonds between carbon atoms. The IUPAC nomenclature system provides a systematic way to name unsaturated hydrocarbons based on their structure and the location of double or triple bonds. Here is the general format for nomenclature of unsaturated hydrocarbons:
Here is an example to illustrate the format:
Compound: 3-methylpent-1-ene
Explanation:
By following the IUPAC nomenclature system, we can assign clear and consistent names to unsaturated hydrocarbons, facilitating effective communication and understanding in the field of organic chemistry.
Nomenclature of Compounds with Functional Groups:
Compounds with functional groups are organic compounds that contain specific groups of atoms that impart characteristic chemical properties. The IUPAC nomenclature system provides guidelines for naming these compounds based on their functional groups. Here is the general format for nomenclature of compounds with functional groups:
Here is an example to illustrate the format:
Compound: 2-methylpropan-1-ol
Explanation:
By following the IUPAC nomenclature system, we can assign clear and consistent names to compounds with functional groups, facilitating effective communication and understanding in the field of organic chemistry.
| Functional Group | Prefix |
|---|---|
| Alcohol | -ol |
| Aldehyde | -al |
| Ketone | -one |
| Carboxylic Acid | -oic acid |
| Ester | -oate |
| Amine | -amine |
| Amide | -amide |
| Nitrile | -nitrile |
| Halide | -ide |
| Ether | -ether |
| Alkene | -ene |
| Alkyne | -yne |
| Aromatic Ring | -benzene |
Lassaigne‘s Test:
Lassaigne‘s test is a chemical test used for the qualitative analysis of organic compounds to detect the presence of nitrogen (N), sulfur (S), and halogens (such as chlorine, bromine, and iodine).
The test involves the fusion of the organic compound with sodium metal, followed by subsequent chemical reactions to detect the specific elements.
Detection of Nitrogen (N):
Chemical Reactions:
2Na + Organic compound →Sodium Fusion
Sodium Fusion + H2O →Sodium Hydroxide (NaOH) + NaHS
NaHS + HCl →NaCl + H2S
NaCl + FeSO4→Prussian Blue coloration
Detection of Sulfur (S):
Chemical Reactions:
2Na + Organic compound →Sodium Fusion
Sodium Fusion + H2O →Sodium Hydroxide (NaOH) + NaHS
NaHS + HCl →NaCl + H2S
NaCl + Pb(CH3COO)2→PbS (Black precipitate)
Detection of Halogens:
Chemical Reactions:
2Na + Organic compound →Sodium Fusion
Sodium Fusion + H2O →Sodium Hydroxide (NaOH) + NaHS
NaHS + HNO3→NaNO3+ H2S
NaNO3+ AgNO3→AgCl (White precipitate), AgBr (Cream precipitate), or AgI (Yellow precipitate)
Lassaigne‘s test provides valuable information about the presence of nitrogen, sulfur, and halogens in organic compounds, aiding in their qualitative analysis.
Isomerism:
Isomerism is a phenomenon in which two or more compounds have the same molecular formula but differ in their structural arrangement or spatial orientation, resulting in distinct chemical and physical properties. Isomers are compounds that exhibit isomerism.
In simpler terms, isomerism refers to the existence of different compounds with the same molecular formula but different arrangements of atoms within the molecule.
Isomerism occurs due to the different ways in which atoms can be connected or arranged in a molecule, leading to variations in the chemical and physical properties of the isomeric compounds.
There are two primary types of isomerism:
Isomerism plays a fundamental role in organic chemistry as it leads to variations in the reactivity, biological activity, and physical properties of compounds. By understanding the different types of isomerism, chemists can study and predict the behavior and properties of isomeric compounds.
Types of Structural Isomerism:
Structural isomerism is a type of isomerism in which compounds have the same molecular formula but differ in the connectivity or arrangement of atoms within the molecule. There are several types of structural isomerism:
These are some of the types of structural isomerism along with their examples. Each type of isomerism results in distinct chemical and physical properties, highlighting the importance of structural isomerism in organic chemistry.
Reaction Mechanism:
Reaction mechanism refers to the step-by-step process that explains how a chemical reaction occurs at the molecular level. It involves the breaking and formation of chemical bonds and the movement of electrons. Understanding the reaction mechanism is essential for predicting the outcome of a reaction and designing new reactions.
Here are some key concepts related to reaction mechanisms:
These concepts play a significant role in understanding how chemical reactions occur, the reactivity of different compounds, and the factors that influence reaction rates and product formation. By studying reaction mechanisms, chemists can gain insights into the underlying principles governing chemical transformations.
Alkanes:
Alkanes are a class of organic compounds that consist exclusively of carbon and hydrogen atoms and are characterized by the presence of single covalent bonds between carbon atoms. They are also known as saturated hydrocarbons because they contain the maximum number of hydrogen atoms possible, with each carbon atom bonded to four other atoms.
Here are some key characteristics and properties of alkanes:
Alkanes serve as important starting materials and solvents in various industrial processes and are also found abundantly in petroleum and natural gas. They provide a foundation for the study of organic chemistry and serve as building blocks for the synthesis of more complex organic compounds.
Hydrogenation of Alkenes:
Alkenes can be hydrogenated to form alkanes by reacting them with hydrogen gas in the presence of a suitable catalyst, such as palladium (Pd), platinum (Pt), or nickel (Ni). The reaction is commonly carried out under high pressure and at elevated temperatures.
Example:
Propene + Hydrogen →Propane
C3H6 + H2 →C3H8
Reduction of Alkyl Halides:
Alkyl halides, such as alkyl chlorides or alkyl bromides, can be reduced to alkanes by using reducing agents like zinc (Zn) and hydrochloric acid (HCl) or lithium aluminum hydride (LiAlH4).
Example:
Ethyl chloride + Zinc/HCl →Ethane
C2H5Cl + Zn/HCl →C2H6
Wurtz Reaction:
The Wurtz reaction is a coupling reaction between alkyl halides and alkyl halides in the presence of sodium (Na) or potassium (K) metal. It leads to the formation of higher alkanes with an even number of carbon atoms.
Example:
2-Methylpropane + Ethyl chloride + Sodium →Pentane
C4H10 + C2H5Cl + 2Na →C5H12
Decarboxylation of Carboxylic Acids:
Carboxylic acids can undergo decarboxylation, where the carboxyl group (-COOH) is removed as carbon dioxide (CO2), resulting in the formation of an alkane. This reaction is usually achieved by heating the carboxylic acid with soda lime (a mixture of sodium hydroxide and calcium oxide).
Example:
Butanoic Acid (Butyric Acid) →Butane
C4H8O2 →C4H10 + CO2
Catalytic Hydrogenation of Unsaturated Hydrocarbons:
Unsaturated hydrocarbons, such as alkenes or alkynes, can be hydrogenated to form alkanes using a suitable catalyst, such as palladium (Pd), platinum (Pt), or nickel (Ni).
Example:
Ethene + Hydrogen →Ethane
C2H4 + H2 →C2H6
Halogenation of Alkanes:
Alkanes can undergo halogenation reactions, where they react with halogens (chlorine, bromine, or iodine) in the presence of heat or light to form alkyl halides.
Example:
Methane + Chlorine →Chloromethane + Hydrogen Chloride
CH4 + Cl2 →CH3Cl + HCl
Nitration of Alkanes:
Alkanes are generally unreactive towards nitration reactions, which involve the addition of a nitro group (-NO2). Alkanes do not readily undergo direct nitration under normal conditions.
Sulfonation of Alkanes:
Alkanes do not undergo direct sulfonation reactions under normal conditions. Sulfonation usually involves the introduction of a sulfonic acid group (-SO3H) into an organic compound.
Oxidation of Alkanes:
Alkanes are relatively unreactive towards oxidation reactions, as they are highly saturated hydrocarbons. They do not readily undergo direct oxidation under normal conditions.
Example:
Methane + Oxygen →Carbon Dioxide + Water
CH4 + 2O2 →CO2 + 2H2O
Applications of Alkanes:
Alkenes:
Alkenes are unsaturated hydrocarbons that contain at least one carbon-carbon double bond. They are characterized by their reactivity and ability to undergo addition reactions. Alkenes play a crucial role in organic chemistry as versatile building blocks for the synthesis of various organic compounds.
General Methods of Preparation of Alkenes:
Alkenes can be prepared by dehydrating alcohols, which involves the removal of water molecule from the alcohol molecule. This can be achieved by heating the alcohol in the presence of a strong acid catalyst, such as concentrated sulfuric acid (H2SO4) or phosphoric acid (H3PO4). The reaction is an example of an elimination reaction, where water is eliminated from the alcohol, leading to the formation of an alkene.
Example:
CH3CH2OH →H2SO4(heat) →CH2=CH2+ H2O
Alkenes can be prepared by the dehalogenation of alkyl halides. This process involves the removal of a halogen atom (such as chlorine or bromine) from an alkyl halide, typically through the use of a reducing agent, such as zinc (Zn) or sodium (Na). The reaction is an example of an elimination reaction, similar to the dehydration of alcohols.
Example:
CH3CH2Br + Zn →CH2=CH2+ ZnBr2
Alkenes can be prepared by the controlled hydrogenation of alkynes. Alkynes are first converted to alkene intermediates through the addition of hydrogen gas (H2) in the presence of a metal catalyst, such as palladium (Pd) or platinum (Pt). The alkene intermediate can then be further hydrogenated to an alkane or isolated as the desired alkene product.
Example:
CH≡CH + H2(Pd/C) →CH2=CH2
Alkenes can be prepared by the dehalogenation of vicinal dihalides, which are compounds with halogen atoms bonded to adjacent carbon atoms. This reaction, known as dehalogenation or dehydrohalogenation, can be achieved by treating the vicinal dihalide with a strong base, such as sodium hydroxide (NaOH) or potassium hydroxide (KOH). The base abstracts a hydrogen atom adjacent to the halogen atoms, resulting in the formation of an alkene.
Example:
CH2Br-CH2Br + KOH →CH2=CH2+ KBr + H2O
Chemical Properties of Alkenes:
Alkenes exhibit various chemical properties due to the presence of the carbon-carbon double bond. One of the notable characteristics of alkenes is their reactivity towards addition reactions, where atoms or groups are added to the carbon-carbon double bond, resulting in the formation of new compounds.
Addition of Hydrogen Halides (Markovnikov‘s Rule):
Alkenes undergo addition reactions with hydrogen halides, such as hydrogen chloride (HCl) or hydrogen bromide (HBr). According to Markovnikov‘s rule, the hydrogen atom of the hydrogen halide adds to the carbon atom of the double bond that already has more hydrogen atoms. This results in the formation of an alkyl halide.
Example:
CH2=CH2+ HCl →CH3CH2Cl
Peroxide Effect:
Alkenes can react differently with hydrogen halides in the presence of peroxides, such as benzoyl peroxide (C6H5C(O)OOC(O)C6H5). In this case, the hydrogen atom adds to the carbon atom of the double bond that already has fewer hydrogen atoms, leading to the formation of a different alkyl halide.
Example:
CH3CH=CH2+ HBr (peroxides) →CH3CHBrCH3
Addition of Water (Hydration):
Alkenes can undergo hydration reactions, where water adds across the carbon-carbon double bond, resulting in the formation of alcohols. This reaction typically occurs in the presence of a strong acid catalyst, such as concentrated sulfuric acid (H2SO4).
Example:
CH2=CH2+ H2O (H2SO4) →CH3CH2OH
Addition of Ozone (Ozonolysis):
Ozonolysis is a reaction in which alkenes react with ozone (O3) to form ozonides. These unstable ozonides are then treated with a reducing agent, such as zinc (Zn) or dimethyl sulfide (CH3)2S, to yield a mixture of aldehydes or ketones, depending on the alkene structure.
Example:
CH3CH=CH2+ O3→CH3COCH2CHO + CH3CHO
Addition of Sulfuric Acid (Sulfonation):
Alkenes can react with concentrated sulfuric acid (H2SO4) to form alkyl hydrogen sulfates. This reaction is called sulfonation and introduces a sulfonic acid group (-SO3H) to the alkene molecule.
Example:
CH2=CH2+ H2SO4→CH3CH2OSO3H
Applications of Alkenes:
Alkenes, with their unique chemical properties and reactivity, find various applications in different fields. Some of the notable applications of alkenes are:
1. Polymer Production:
Alkenes are extensively used in the production of polymers, such as polyethylene and polypropylene. These polymers have a wide range of applications in industries including packaging, textiles, automotive, construction, and more. The ability of alkenes to undergo polymerization reactions enables the formation of long-chain polymers with desirable properties.
2. Solvent Production:
Certain alkenes, such as ethene (ethylene) and propene (propylene), are used as solvents in various industrial processes. These alkenes serve as excellent solvents for dissolving and extracting substances in chemical reactions, cleaning, and degreasing operations.
3. Fuel and Energy:
Alkenes are crucial in the fuel and energy sector. Ethene and propene are used as feedstocks for the production of gasoline, diesel, and other fuels through processes like cracking and reforming. Additionally, alkenes can be converted into biofuels through bioconversion techniques, contributing to sustainable energy sources.
4. Chemical Synthesis:
Alkenes serve as versatile building blocks in chemical synthesis. They undergo a wide range of reactions, such as addition, oxidation, and reduction, allowing the synthesis of various organic compounds. Alkenes are utilized in the synthesis of pharmaceuticals, agrochemicals, fragrances, dyes, and many other specialty chemicals.
5. Manufacturing of Plastics and Fibers:
Alkenes play a vital role in the manufacturing of plastics and synthetic fibers. Polyethylene and polypropylene, derived from alkenes, are widely used in the production of bottles, containers, pipes, films, and fibers for textiles. These materials offer durability, flexibility, and resistance to chemicals.
6. Adhesive and Sealant Production:
Some alkenes, like ethylene and propylene, are used in the production of adhesives and sealants. These materials provide strong bonding and sealing properties and are utilized in industries such as construction, automotive, and packaging.
7. Agricultural Applications:
Alkenes find applications in agriculture as plant growth regulators and in the production of herbicides and pesticides. Ethene is used to control fruit ripening and promote uniform flowering in plants, while certain alkenes are incorporated into herbicides to control weed growth effectively.
8. Research and Development:
Alkenes are of significant interest in research and development, particularly in the field of organic chemistry. Their reactivity and ability to undergo various transformations make them valuable tools for studying reaction mechanisms, designing new catalysts, and developing novel organic compounds.
These are just a few examples of the applications of alkenes. Their versatility and importance extend across numerous industries, contributing to advancements in materials, energy, pharmaceuticals, and many other areas.
Alkynes:
Alkynes are a class of unsaturated hydrocarbons that contain a carbon-carbon triple bond. They are characterized by their reactivity and unique chemical properties. Alkynes play a crucial role in organic synthesis and have various applications in different industries.
General Methods of Preparation of Alkynes:
There are several methods for the preparation of alkynes. Here are some commonly used methods:
1.Direct Combination of Elements:
Alkynes can be prepared by the direct combination of carbon and hydrogen atoms. This method is commonly used in the laboratory for the synthesis of simple alkynes. For example:
2C(s) + H2(g) →HC≡CH(g) (Ethyne)
2.Dehydrohalogenation of Vicinal Dihalides:
Alkynes can be synthesized by the dehydrohalogenation of vicinal dihalides (compounds with two halogen atoms on adjacent carbon atoms) using a strong base. The elimination of two halogen atoms results in the formation of a triple bond. For example:
CH3CHClCH2Cl + 2KOH(aq) →CH≡CCH + 2KCl + 2H2O
3.Heating Trihalogen Derivatives with Silver Powder:
Alkynes can be prepared by heating trihalogen derivatives (compounds with three halogen atoms) with silver powder. The silver powder acts as a reducing agent, removing the halogen atoms and forming a triple bond. For example:
CH3CCl3+ 6Ag →HC≡CH + 3AgCl + 2AgCl2
These methods provide a general overview of how alkynes can be prepared. Each method offers advantages and may be suitable for specific applications or desired products.
Chemical Properties of Alkynes:
Alkynes exhibit various chemical reactions due to the presence of a carbon-carbon triple bond. Here are some important chemical properties of alkynes:
1.Addition of Hydrogen:
Alkynes can undergo addition reactions with hydrogen (H2) in the presence of a catalyst such as palladium (Pd) or platinum (Pt) to form alkanes. This reaction is known as hydrogenation. For example:
HC≡CH + H2→CH2=CH2
2.Addition of HX (Hydrogen Halides):
Alkynes react with hydrogen halides (HX) such as hydrochloric acid (HCl) or hydrobromic acid (HBr) to form vinyl halides. The addition follows Markovnikov‘s rule, where the halogen atom attaches to the carbon atom with fewer hydrogen atoms. For example:
HC≡CH + HBr →CH2=CHBr
3.Addition of Water (Hydration):
Alkynes can undergo hydration, where water (H2O) adds across the triple bond in the presence of an acid catalyst to form ketones. For example:
HC≡CH + H2O →CH3C=O
4.Action with Sodium:
Alkynes react with sodium (Na) to form sodium alkynides. This reaction involves the replacement of a hydrogen atom in the alkyne by a sodium atom. For example:
2 HC≡CH + 2Na →2NaC≡CNa + H2
5.Action with Ammonical Silver Nitrate Solution:
Alkynes react with ammonical silver nitrate (AgNO3+ NH3) to form a silver acetylide precipitate, which is explosive and highly sensitive to shock. This reaction can be used for the detection of alkynes. For example:
2 HC≡CH + 2AgNO3+ 2NH3→2AgC≡CAg + 2NH4NO3
6.Action with Ammonical Cuprous Chloride (Fehling‘s Solution):
Alkynes react with ammonical cuprous chloride (CuCl2+ NH3) to form a red precipitate of copper acetylide. This reaction is used for the detection of alkynes. For example:
2 HC≡CH + 2CuCl2+ 2NH3→2CuC≡CCu + 2NH4Cl
These are some of the important chemical reactions exhibited by alkynes. These reactions demonstrate the versatility of alkynes and their ability to undergo diverse transformations.
Acidity of Alkynes:
Alkynes are weakly acidic due to the presence of a sp hybridized carbon atom in the triple bond. The acidity of alkynes is attributed to the polarizability of the carbon-hydrogen (C-H) bond, which allows for the stabilization of the resulting carbanion after deprotonation. The acidity of alkynes increases with the presence of electron-withdrawing groups (EWG) or increased alkyl substitution on the carbon atom bearing the acidic hydrogen.
Applications of Alkynes:
Alkynes find applications in various fields due to their unique chemical properties and reactivity. Here are some notable applications:
1.Chemical Synthesis:
Alkynes serve as versatile building blocks in organic synthesis. They can undergo various reactions such as addition, oxidation, reduction, and coupling reactions to produce a wide range of organic compounds. The ability of alkynes to form carbon-carbon triple bonds provides synthetic chemists with opportunities to create complex molecular structures.
2.Industrial Processes:
Alkynes are used in industrial processes such as polymerization and manufacturing of specialty chemicals. For example, acetylene (ethyne) is an important feedstock in the production of plastics, synthetic fibers, and rubber. It is also used as a fuel gas for welding and cutting.
3.Pharmaceuticals:
Alkynes play a crucial role in pharmaceutical research and development. They serve as key intermediates in the synthesis of pharmaceutical drugs and bioactive compounds. The presence of a triple bond in alkynes allows for the introduction of specific functional groups and structural modifications to enhance the biological activity of molecules.
4.Organometallic Chemistry:
Alkynes are widely used in organometallic chemistry as ligands to form transition metal complexes. These complexes exhibit unique catalytic properties and are employed in various catalytic processes, including cross-coupling reactions, hydrogenation, and carbonylation reactions.
5.Research and Development:
Alkynes continue to be of great interest in scientific research and development. Their reactivity and ability to form complex molecular architectures make them valuable tools in exploring new reaction pathways, developing novel materials, and advancing our understanding of chemical bonding and reactivity.
These are just a few examples of the applications of alkynes. The versatility and reactivity of alkynes make them valuable compounds in various industries and scientific endeavors.
Kolbe‘s Electrolytic Methods:
Kolbe‘s electrolytic methods are used for the preparation of alkane, alkene, and alkyne compounds by the electrolysis of sodium or potassium salts of carboxylic acids. These methods involve the use of an electrolytic cell and specific conditions to achieve the desired product. Here are the details of each method:
1.Preparation of Alkane:
In Kolbe‘s electrolytic method, alkane compounds can be prepared by the electrolysis of the sodium or potassium salts of carboxylic acids. The process involves the following steps:
2CH3COO-(aq) - 2e- -> 2CH3COOH (aq) + CO2(g)
CH3COOH (aq) -> CH3COO+(aq) + H+(aq)
CH3COO+(aq) + H2O (l) -> CH3CH3(g) + H3O+(aq)
This method allows for the preparation of various alkanes by choosing different carboxylic acids as starting materials.
2.Preparation of Alkene:
Kolbe‘s electrolytic method can also be used for the preparation of alkene compounds. The process is similar to the preparation of alkanes but involves an additional step:
CH3COO+ (aq) -> CH2=CH2(g) + CO2(g) + H+(aq)
This method provides a way to selectively prepare specific alkene compounds by using appropriate carboxylic acids.
3.Preparation of Alkyne:
Similarly, Kolbe‘s electrolytic method can be employed for the preparation of alkyne compounds by incorporating an additional step:
CH3COO+ (aq) -> HC≡CH (g) + CO2(g) + H+(aq)
This method allows for the synthesis of various alkyne compounds by selecting appropriate carboxylic acids as starting materials.
Kolbe‘s electrolytic methods provide useful routes for the preparation of alkane, alkene, and alkyne compounds from carboxylic acids. These methods offer a convenient and efficient way to access a wide range of hydrocarbon compounds.
Introduction to Aromatic Compounds:
Aromatic compounds are a class of organic compounds that exhibit unique chemical and physical properties due to the presence of an aromatic ring structure. The term "aromatic" originally referred to compounds with pleasant odors, but it was later used to describe compounds containing a specific type of cyclic conjugated system known as an aromatic ring.
The aromatic ring, also known as a benzene ring, consists of a planar hexagonal structure with alternating single and double bonds between carbon atoms. The most common aromatic compound is benzene (C6H6), which serves as the basis for the aromatic nature of other compounds.
Characteristics of Aromatic Compounds:
Aromatic compounds possess several distinct characteristics that differentiate them from other organic compounds:
These characteristics make aromatic compounds of great importance in various areas of chemistry, including organic synthesis, pharmaceuticals, dyes, fragrances, and materials science.
Hückel‘s Rule of Aromaticity:
Hückel‘s rule is a criterion used to determine whether a cyclic compound with a conjugated pi electron system is aromatic. It was proposed by Erich Hückel, a German physicist and chemist, in 1931. According to Hückel‘s rule, a compound must meet specific criteria to be considered aromatic:
If a compound fulfills all these criteria, it is considered aromatic. This means that it exhibits enhanced stability and unique reactivity due to the delocalization of pi electrons.
It‘s important to note that compounds that do not satisfy Hückel‘s rule are considered non-aromatic or antiaromatic. Antiaromatic compounds have different electronic properties and tend to be less stable than their aromatic counterparts.
Hückel‘s rule has been successfully applied to many aromatic compounds, including benzene (C6H6), pyrrole (C4H5N), and furan (C4H4O), among others. Understanding the aromaticity based on Hückel‘s rule is essential in the field of organic chemistry and provides insights into the behavior of aromatic compounds.
Kekulé‘s Structure of Benzene:
The Kekulé structure of benzene is a historical representation proposed by Friedrich August Kekulé in 1865 to explain the structure of the benzene molecule. Prior to Kekulé‘s proposal, the structure of benzene was not well understood.
Kekulé‘s structure suggests that benzene (C6H6) is a cyclic molecule with six carbon atoms and six hydrogen atoms. He proposed that benzene consists of a hexagonal ring of carbon atoms, with alternating single and double bonds between them.
The alternating single and double bonds in the Kekulé structure can be represented as:
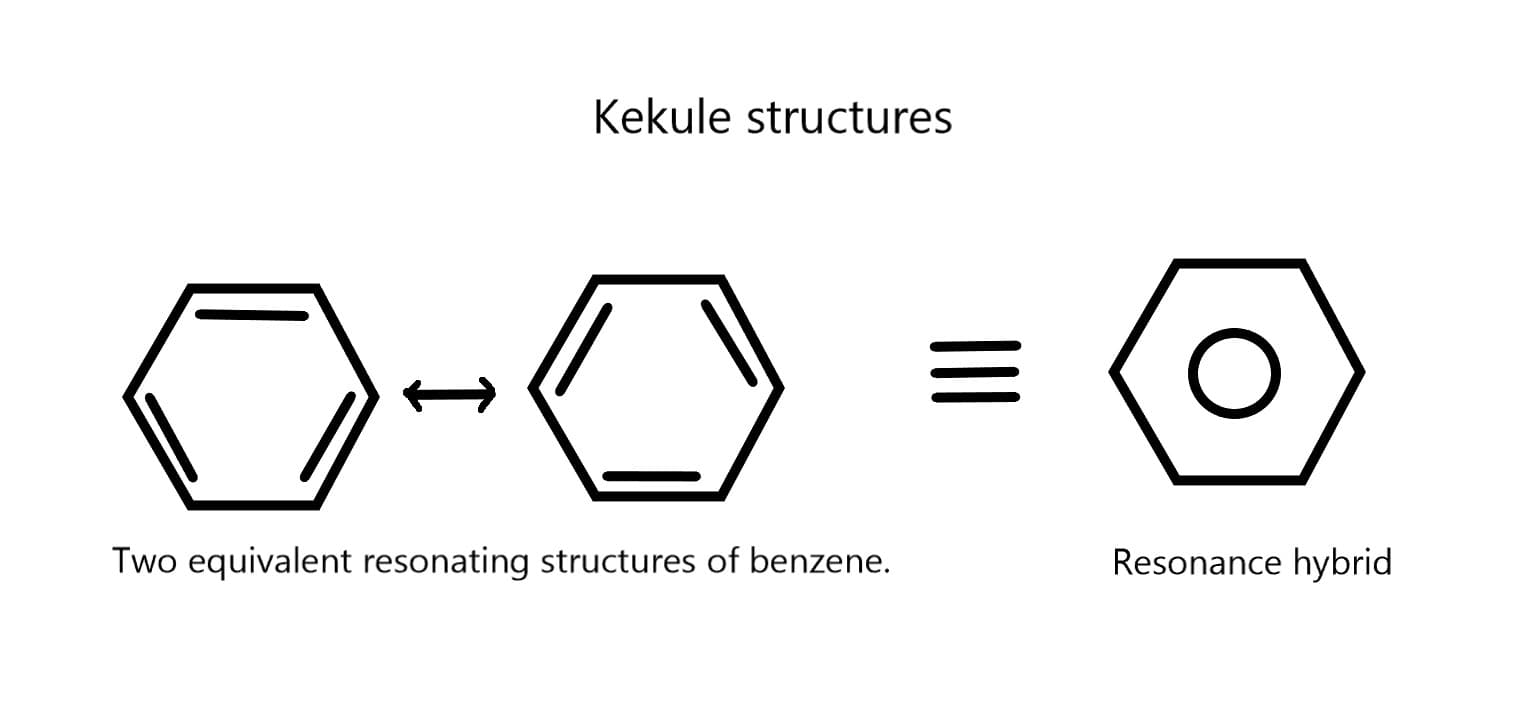
According to Kekulé, the structure of benzene undergoes dynamic oscillation, where the double bonds continuously shift around the ring. This oscillation explained the observed chemical behavior of benzene, including its resistance to addition reactions and the presence of substitution reactions.
While Kekulé‘s structure was a significant breakthrough in understanding the nature of benzene, it was later refined with the concept of delocalized pi electron density. The actual structure of benzene is best described by a resonance hybrid, where the six pi electrons are delocalized over the entire ring rather than localized in specific double bonds.
Kekulé‘s structure of benzene laid the foundation for further studies on aromatic compounds and had a profound impact on the development of organic chemistry.
Resonance Structure of Benzene:
Benzene is best represented by a resonance hybrid structure due to its delocalized pi electrons. The resonance structure illustrates the distribution of pi electron density throughout the benzene ring. In the resonance structure, the double bonds are not fixed between specific carbon atoms but instead continuously shift around the ring.
One of the resonance structures of benzene can be represented as:

In this structure, the double bonds are shown between different pairs of carbon atoms, indicating the possibility of electron delocalization. However, it‘s important to note that the actual electronic structure of benzene is a combination of all the possible resonance structures.
The resonance hybrid of benzene represents the delocalization of six pi electrons over the entire ring, resulting in a more stable and lower energy system compared to individual localized double bonds. This delocalization gives benzene its unique stability and characteristic properties, such as its resistance to addition reactions and enhanced reactivity in substitution reactions.
The resonance structure of benzene highlights the concept of electron delocalization and the importance of considering multiple contributing structures when describing the behavior of aromatic compounds.
Preparation of Benzene:
Benzene can be prepared from various precursor compounds, including phenol, acetylene, and sodium benzoate. Here are the methods for the preparation of benzene from these compounds:
These methods provide different routes for the preparation of benzene from phenol, acetylene, and sodium benzoate, offering flexibility in obtaining this important aromatic compound for various applications.
Preparation of Benzene from Chlorobenzene:
Benzene can be prepared from chlorobenzene through a process calledhydrodechlorination. The hydrodechlorination reaction involves the removal of a chlorine atom from the chlorobenzene molecule, resulting in the formation of benzene. This reaction is typically carried out in the presence of a reducing agent, such as zinc (Zn), and a suitable solvent, such as ethanol (C2H5OH).
The reaction can be represented as follows:
C6H5Cl + Zn →C6H6+ ZnCl2
In this reaction, the zinc (Zn) acts as a reducing agent and donates electrons to the chlorine atom, resulting in the formation of zinc chloride (ZnCl2) as a byproduct. The removal of the chlorine atom from chlorobenzene transforms it into benzene.
Hydrodechlorination is an important method for converting chlorobenzene, which is a halogenated aromatic compound, into benzene, which is a widely used aromatic compound with various applications in the chemical industry.
Physical Properties of Benzene:
Benzene is a colorless liquid with a distinct sweet aroma. It has the chemical formula C6H6and a molecular weight of 78.11 g/mol. Here are some key physical properties of benzene:
These physical properties contribute to the unique characteristics and behavior of benzene in various applications, including its use as a solvent, chemical intermediate, and fuel additive.
Chemical Reactions of Benzene:
Benzene, being an aromatic compound, undergoes several characteristic chemical reactions. Here are some important reactions of benzene:
C6H6+ Cl2→C6H5Cl + HCl
C6H6+ HNO3→C6H5NO2+ H2O
C6H6+ H2SO4→C6H5SO3H + H2O
C6H6+ CH3Cl →C6H5CH3+ HCl
C6H6+ 3H2→C6H12
C6H6+ Br2→C6H6Br2
C6H6+ 3O3→C6H5COOH + 3O2
C6H6+ 15/2O2→6CO2+ 3H2O
These are some of the important chemical reactions of benzene that highlight its reactivity and versatility in various synthetic processes and industrial applications.
In benzene derivatives, the orientation of substituent groups is determined by the principles of ortho-, meta-, and para-substitution. These terms describe the relative positions of substituents on the benzene ring:
1. Ortho-:In ortho-substitution, the substituent group occupies an adjacent position to the substituent already present on the benzene ring. The ortho position is denoted as o-.
2. Meta-:In meta-substitution, the substituent group occupies a position that is two carbons away from the existing substituent on the benzene ring. The meta position is denoted as m-.
3. Para-:In para-substitution, the substituent group occupies a position that is opposite to the existing substituent on the benzene ring. The para position is denoted as p-.
The orientation of substituents in benzene derivatives is primarily governed by steric and electronic factors. Steric hindrance and electronic effects such as resonance and inductive effects play a significant role in determining the preferred orientation of substituents.
Here are some general guidelines for the orientation of substituents:
1. Ortho- and para- directors:Substituents that have electron-donating groups or lone pairs of electrons are ortho- and para- directors. These groups facilitate the electrophilic substitution of benzene at the ortho and para positions. Examples of ortho- and para- directing groups include alkyl groups (-CH3), hydroxyl group (-OH), amino group (-NH2), and methoxy group (-OCH3).
2. Meta- directors:Substituents that have electron-withdrawing groups tend to be meta- directors. These groups direct the electrophilic substitution of benzene at the meta position. Examples of meta- directing groups include nitro group (-NO2), carbonyl group (-C=O), and halogens (-F, -Cl, -Br, -I).
It‘s important to note that these guidelines are general and may have exceptions depending on the specific substituents and reaction conditions. Additionally, multiple substituents can influence each other‘s orientation, leading to more complex substitution patterns.
Understanding the orientation of substituents in benzene derivatives is crucial in predicting the products of electrophilic aromatic substitution reactions and studying the reactivity and properties of aromatic compounds.
Benzene is a versatile compound with numerous applications in various industries. Some of the common uses of benzene include:
1. Chemical Intermediates:Benzene is widely used as a starting material or intermediate in the production of various chemicals. It serves as a precursor for the synthesis of numerous compounds, including plastics, dyes, detergents, pharmaceuticals, and synthetic fibers.
2. Solvent:Benzene has excellent solvent properties and is used as a solvent for many organic substances, such as resins, oils, waxes, rubber, and certain polymers. It is particularly effective in dissolving nonpolar compounds.
3. Fuel:Benzene can be used as a component in gasoline fuels due to its high energy content. However, its use as a fuel additive has declined due to environmental concerns and health risks associated with its toxicity.
4. Rubber and Tire Manufacturing:Benzene is an essential ingredient in the production of synthetic rubber, which is used in the manufacturing of tires, gaskets, belts, and various other rubber products.
5. Pharmaceuticals:Benzene and its derivatives are widely utilized in the pharmaceutical industry for the synthesis of drugs and active pharmaceutical ingredients (APIs). Many medications and therapeutic compounds contain benzene-based structures.
6. Plastics and Polymers:Benzene is a key component in the production of various plastics and polymers, including polystyrene, nylon, polyurethane, and polyester. These materials find applications in a wide range of industries, from packaging to automotive parts.
7. Paints and Coatings:Benzene is used in the formulation of paints, varnishes, and coatings due to its solvent properties and ability to dissolve pigments and resins. It contributes to the proper application and drying of these products.
8. Adhesives and Sealants:Benzene-based compounds are commonly employed in the production of adhesives, sealants, and bonding agents. They provide strong adhesion properties and are utilized in various industries, including construction, automotive, and electronics.
9. Aromatic Hydrocarbon Production:Benzene is a fundamental building block in the production of other aromatic hydrocarbons, such as toluene, xylene, and ethylbenzene. These compounds have numerous industrial applications in solvents, fuels, and chemical synthesis.
10. Laboratory Reagent:Benzene is used as a laboratory reagent for various purposes, including chemical reactions, extraction processes, and as a solvent for analytical techniques.
It‘s important to note that benzene is a toxic substance and poses health risks. Therefore, its use and handling should strictly adhere to safety regulations and guidelines to minimize exposure and ensure proper precautions are taken.
Chemical Industry and its Importance:
The chemical industry plays a crucial role in modern society by producing a wide range of chemicals and materials that are essential for various sectors, including agriculture, healthcare, energy, transportation, manufacturing, and consumer goods. It involves the production, processing, and transformation of raw materials into valuable products through chemical reactions and processes.
The chemical industry contributes to economic growth, job creation, and technological advancements. It provides essential materials for construction, fuels for energy production, fertilizers for agriculture, pharmaceuticals for healthcare, and various chemicals for industrial processes. The industry‘s innovations and advancements drive progress and improve the quality of life.
The Stages in Producing a New Product:
Producing a new product in the chemical industry involves several stages:
1. Research and Development (R&D): This stage involves conducting research, exploring new ideas, and developing concepts for new products or processes. R&D aims to discover innovative solutions, optimize existing products, and ensure safety and regulatory compliance.
2. Pilot Plant Testing: Once a product concept is developed, it moves to pilot plant testing. Small-scale prototypes are produced to evaluate the feasibility, scalability, and performance of the product or process under controlled conditions.
3. Scale-Up: If the pilot plant testing is successful, the product or process is scaled up for commercial production. This stage involves optimizing the process parameters, equipment selection, and ensuring efficiency and cost-effectiveness.
4. Production: In this stage, the product is manufactured on a larger scale using established processes and equipment. Quality control measures are implemented to ensure consistency and meet product specifications.
5. Marketing and Distribution: Once the product is manufactured, it is marketed and distributed to customers and end-users through appropriate channels.
Economics of Production:
The economics of production in the chemical industry involve various factors:
- Cost of raw materials: Raw materials constitute a significant portion of the production costs. The availability, prices, and sourcing of raw materials impact the overall economics of production.
- Process efficiency: Efficient production processes that maximize yield, minimize waste, and optimize energy usage contribute to cost-effectiveness.
- Equipment and infrastructure: Capital investment in equipment, infrastructure, and maintenance impacts production costs and efficiency.
- Labor and operational costs: Labor costs, including wages, training, and safety measures, as well as operational expenses, such as utilities and maintenance, affect the economics of production.
- Market demand and pricing: Market demand, competition, and pricing dynamics influence the profitability of production.
Cash Flow in the Production Cycle:
The production cycle in the chemical industry involves cash flow considerations:
- Initial investment: Capital investment is required for setting up the production plant, acquiring equipment, and conducting R&D.
- Operating costs: Ongoing operational expenses, such as raw materials, labor, utilities, and maintenance, impact cash flow.
- Revenue generation: Sales and revenue from the products produced contribute to cash flow.
- Return on investment: Profitability and return on investment are essential factors in sustaining the cash flow and ensuring the financial viability of the production cycle.
Running a Chemical Plant:
Running a chemical plant involves various aspects:
- Process control: Monitoring and controlling the chemical processes to ensure safe and efficient operation.
- Safety measures: Implementing rigorous safety protocols and measures to protect employees, the environment, and the surrounding community.
- Maintenance and repairs: Regular maintenance, inspections, and repairs of equipment and infrastructure to ensure reliability and prevent downtime.
- Supply chain management: Efficient management of raw material sourcing, inventory, and logistics to ensure uninterrupted production.
- Quality control: Implementing quality control measures to meet product specifications and regulatory standards.
- Environmental compliance: Ensuring compliance with environmental regulations, waste management, and emission control measures.
Designing a Chemical Plant:
Designing a chemical plant involves:
- Process design: Designing the chemical processes, selecting reaction pathways, and determining process parameters.
- Equipment selection: Selecting appropriate equipment, such as reactors, distillation columns, pumps, and control systems, based on process requirements.
- Safety considerations: Incorporating safety features, emergency response systems, and process control measures to ensure safe operation.
- Efficiency and optimization: Designing the plant layout to maximize efficiency, minimize energy usage, and optimize production flow.
- Regulatory compliance: Ensuring compliance with local, national, and international regulations and standards.
Continuous and Batch Processing:
- Continuous processing: In continuous processing, the production runs continuously without interruptions. The raw materials are continuously fed into the process, and the products are continuously collected. This method allows for high-volume production and efficient use of resources.
- Batch processing: In batch processing, the production occurs in discrete batches. Each batch follows a specific set of operations, starting with raw material addition and ending with the product collection. Batch processing is suitable for smaller-scale productions, customizations, and products with specific requirements.
Environmental Impact of the Chemical Industry:
The chemical industry has both positive and negative environmental impacts:
- Positive impact: The chemical industry contributes to sustainable development by providing solutions for renewable energy, water treatment, pollution control, and sustainable agriculture.
- Negative impact: Chemical production can generate waste, emissions, and pollutants. Proper waste management, emission controls, and environmental regulations are crucial in minimizing the negative environmental impact.
- Sustainable practices: The industry is increasingly adopting sustainable practices, such as green chemistry, recycling, resource efficiency, and renewable feedstocks, to minimize environmental impact.
Preparation of Ammonia from Haber & lsquo;s Process:
Principle:
The Haber‘s process is a method for the industrial production of ammonia. It involves the catalytic reaction between nitrogen gas (N2) and hydrogen gas (H2) under high pressure and moderate temperature. The reaction is exothermic and reversible, and it is carried out in the presence of an iron catalyst.
Flow sheet diagram:
N2(g) + 3H2(g) ⇌ 2NH3(g)
Notes:
- The Haber‘s process is conducted in a reactor vessel equipped with a catalyst bed of iron.
- Nitrogen gas (N2) and hydrogen gas (H2) are compressed and fed into the reactor.
- The reaction is carried out at a high pressure of around 200-300 atmospheres to favor the forward reaction and maximize the ammonia yield.
- The temperature is typically maintained at around 400-500°C, which is a compromise between achieving a reasonable reaction rate and minimizing the reverse reaction.
- The reaction mixture is cooled to condense the ammonia gas, which is then collected and purified.
- The unreacted nitrogen and hydrogen gases are recycled back into the reactor to improve the efficiency of the process.
- The ammonia produced is used as a raw material for various applications, including the production of fertilizers, explosives, and chemicals.
- The Haber‘s process is a crucial industrial process that has significantly contributed to the availability of ammonia for agricultural and industrial purposes.
Preparation of Nitric Acid by Ostwald‘s Process:
Principle:
Ostwald‘s process is a method for the industrial production of nitric acid (HNO3). It involves the catalytic oxidation of ammonia (NH3) to form nitric oxide (NO), followed by the further oxidation of nitric oxide to form nitrogen dioxide (NO2). The nitrogen dioxide is then absorbed in water to produce nitric acid.
Flow sheet diagram:
NH3(g) + 5/4O2(g) →NO (g) + 3/2H2O (g)
2NO (g) + O2(g) →2NO2(g)
3NO2(g) + H2O (l) →2HNO3(aq) + NO (g)
Notes:
- In Ostwald‘s process, ammonia (NH3) is oxidized by air or oxygen in the presence of a platinum or rhodium catalyst in the first step, forming nitric oxide (NO) and water vapor.
- The formed nitric oxide is then further oxidized to nitrogen dioxide (NO2) by reacting with more oxygen.
- Nitrogen dioxide is a reddish-brown gas that is dissolved in water to produce nitric acid (HNO3).
- The nitric acid is typically obtained as a solution with a concentration of around 68-70%.
- The reaction between nitrogen dioxide and water is highly exothermic and needs to be carefully controlled to prevent explosions.
- The nitric acid produced is widely used in the production of fertilizers, explosives, dyes, and various organic and inorganic compounds.
- Ostwald‘s process is an important industrial process for the large-scale production of nitric acid, which is a vital chemical in many industries.
Manufacture of Sulfuric Acid by Contact Process:
Principle:
The contact process is a method for the industrial production of sulfuric acid (H2SO4). It involves the catalytic oxidation of sulfur dioxide (SO2) to form sulfur trioxide (SO3), followed by the absorption of sulfur trioxide in water to produce sulfuric acid.
Flow sheet diagram:
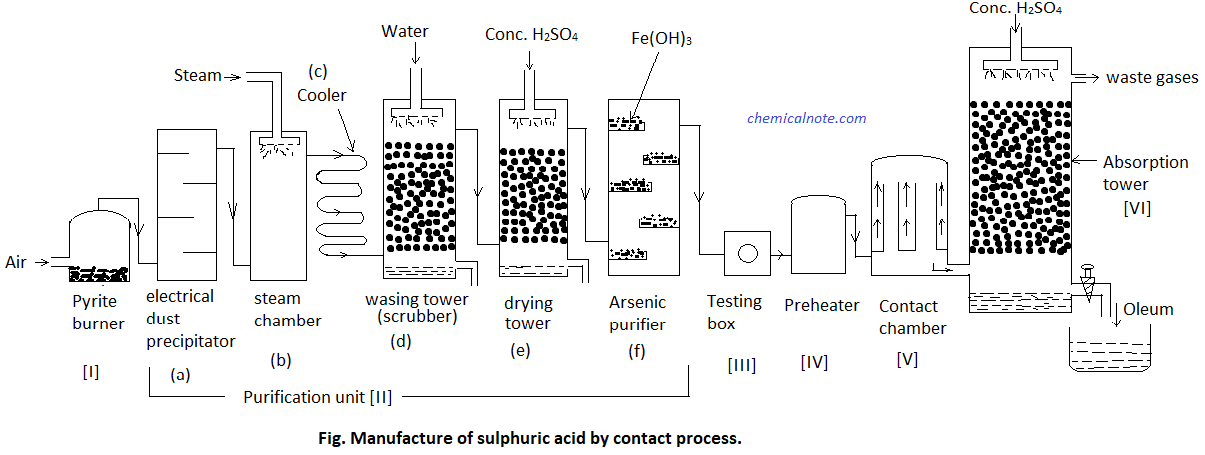
2SO2(g) + O2(g) →2SO3(g)
SO3(g) + H2O (l) →H2SO4(l)
Notes:
- In the contact process, sulfur dioxide (SO2) is obtained from the burning of sulfur or from sulfide ores, such as pyrite (FeS2).
- The sulfur dioxide is then oxidized to sulfur trioxide (SO3) by reacting with excess oxygen in the presence of a vanadium(V) oxide (V2O5) catalyst at a temperature of around 450-500°C.
- The produced sulfur trioxide is a highly reactive and volatile compound, which is then absorbed in concentrated sulfuric acid to form oleum (a solution of sulfur trioxide in sulfuric acid).
- The oleum is then diluted with water to produce sulfuric acid of the desired concentration.
- The contact process is carried out in several stages with heat recovery to optimize energy efficiency.
- Sulfuric acid is a strong acid widely used in various industries, including fertilizers, dyes, detergents, pharmaceuticals, and chemical manufacturing.
- The contact process is the most commonly used method for the commercial production of sulfuric acid due to its efficiency and high yield.
Manufacture of Sodium Hydroxide by Diaphragm Cell:
Principle:
The diaphragm cell process is a method for the industrial production of sodium hydroxide (NaOH) and chlorine gas (Cl2). It involves the electrolysis of a sodium chloride (NaCl) solution using a diaphragm to separate the anode and cathode compartments, preventing the mixing of the produced chlorine and sodium hydroxide.
Flow sheet diagram:
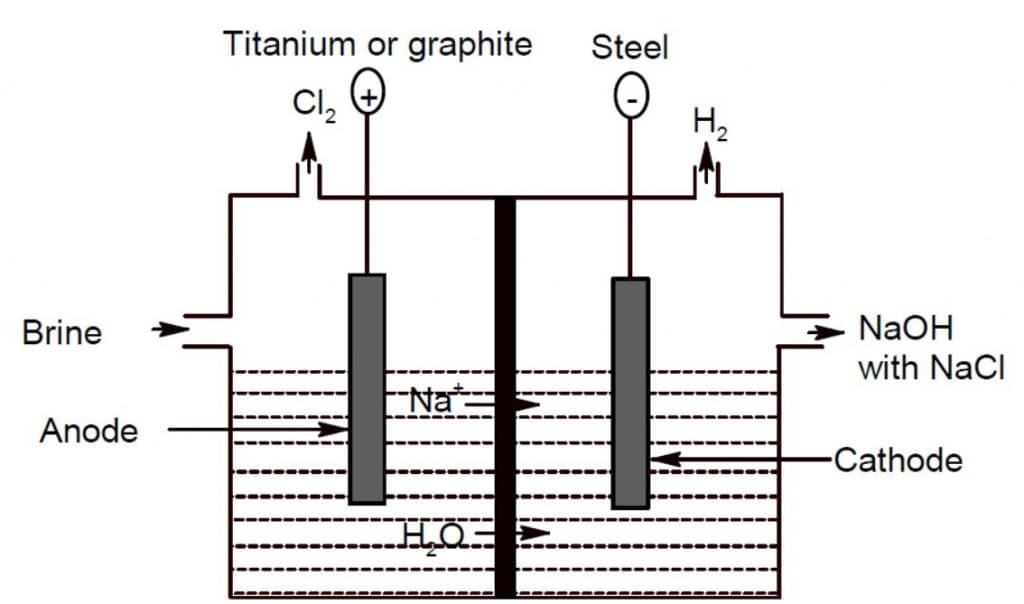
At Anode (oxidation): 2Cl-(aq) →Cl2(g) + 2e-
At Cathode (reduction): 2H2O(l) + 2e-→H2(g) + 2OH-(aq)
Overall Reaction: 2NaCl(aq) + 2H2O(l) →Cl2(g) + H2(g) + 2NaOH(aq)
Notes:
- In the diaphragm cell process, a concentrated sodium chloride (NaCl) solution, known as brine, is fed into the electrolytic cell.
- The electrolytic cell consists of an anode and a cathode compartment separated by a diaphragm made of asbestos or other materials.
- Direct current (DC) is passed through the cell, causing the oxidation of chloride ions (Cl-) at the anode, releasing chlorine gas (Cl2).
- At the cathode, water molecules (H2O) are reduced, producing hydrogen gas (H2) and hydroxide ions (OH-).
- The hydroxide ions combine with sodium ions (Na+) from the sodium chloride solution to form sodium hydroxide (NaOH).
- The chlorine gas and sodium hydroxide are continuously removed from their respective compartments.
- The diaphragm prevents the mixing of chlorine and sodium hydroxide, allowing their separate collection.
- Sodium hydroxide, also known as caustic soda, is a strong base used in various industries, including chemical manufacturing, pulp and paper, detergents, and water treatment.
- The diaphragm cell process is a widely used and efficient method for the commercial production of sodium hydroxide and chlorine gas.
Manufacture of Sodium Carbonate by Solvay or Ammonia Soda Process:
Principle:
The Solvay or Ammonia Soda process is a method for the industrial production of sodium carbonate (Na2CO3) from sodium chloride (NaCl) and limestone (CaCO3). It involves a series of chemical reactions that utilize ammonia and carbon dioxide to convert sodium chloride into sodium carbonate.
Flow sheet diagram:
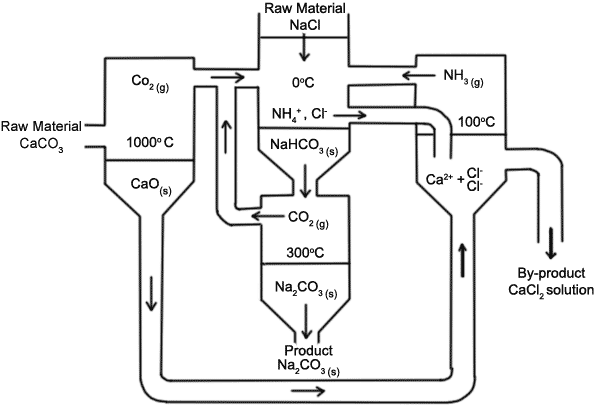
Step 1: Preparation of Ammonia
Step 2: Formation of Ammonium Chloride
Step 3: Formation of Sodium Hydrogen Carbonate
Step 4: Formation of Sodium Carbonate
Overall Reaction: 2NaCl(aq) + CaCO3(s) + H2O(l) + NH3(g) + CO2(g) →2NaHCO3(aq) + CaCl2(aq)
Notes:
- The Solvay or Ammonia Soda process begins with the preparation of ammonia (NH3) from a nitrogen source and hydrogen gas.
- The ammonia is then combined with carbon dioxide (CO2) and water (H2O) to form ammonium bicarbonate (NH4HCO3).
- In the presence of calcium carbonate (limestone), the ammonium bicarbonate reacts to form sodium hydrogen carbonate (NaHCO3) and calcium chloride (CaCl2).
- The sodium hydrogen carbonate solution is then heated, causing the release of carbon dioxide gas and the formation of sodium carbonate (Na2CO3).
- The carbon dioxide released in the previous step is recycled back to the process and used in the formation of ammonium bicarbonate.
- The sodium carbonate solution obtained is concentrated and crystallized to produce solid sodium carbonate.
- Sodium carbonate, also known as soda ash or washing soda, is a versatile compound used in various industries, including glass manufacturing, detergents, water treatment, and chemical processes.
- The Solvay or Ammonia Soda process is an important method for the commercial production of sodium carbonate and has been widely used since its development in the 19th century by Ernest Solvay.
Chemical Fertilizers:
Chemical fertilizers, also known as inorganic fertilizers, are synthetic substances that provide essential nutrients to plants for their optimal growth and development. They are widely used in agriculture and gardening to supplement nutrient levels in the soil and enhance crop productivity. Here are some commonly used chemical fertilizers:
Chemical fertilizers provide readily available nutrients to plants, promoting healthy growth and increasing crop yields. However, their excessive and improper use can lead to environmental pollution and soil degradation, highlighting the importance of responsible fertilizer application and nutrient management practices.
Manufacture of Urea from Ammonium Carbamate:
The production of urea involves the conversion of ammonium carbamate, a precursor compound, into urea through a two-step process. Here are the key steps involved:
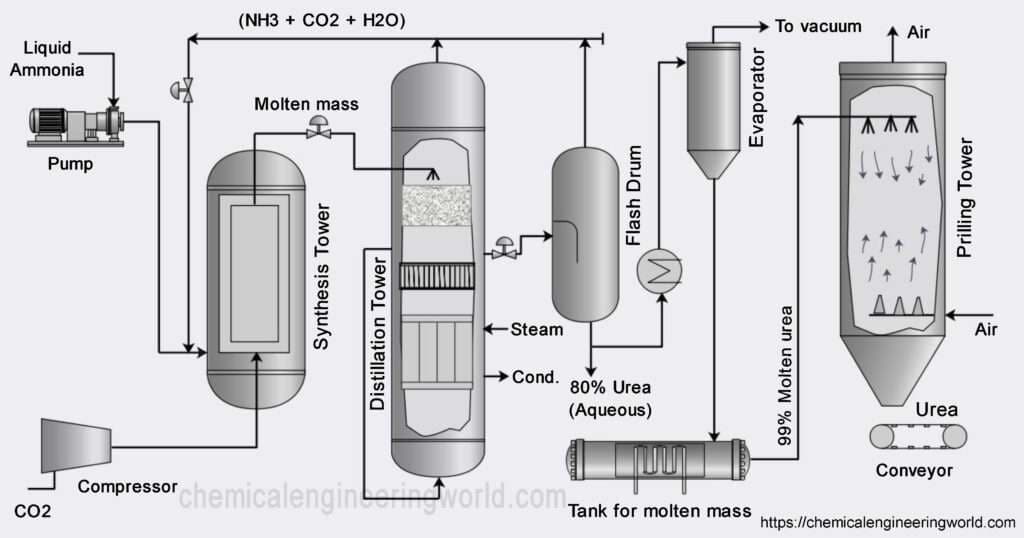
The overall reaction can be represented as follows:
2NH3+ CO2→NH2COONH4
NH2COONH4→CO(NH2)2+ H2O
The urea produced is then further purified and processed to obtain the desired commercial-grade urea fertilizer. It is typically in the form of white, crystalline granules that are soluble in water.
The manufacture of urea from ammonium carbamate is a crucial process in the fertilizer industry, as urea is one of the most widely used nitrogen fertilizers globally.